
eBook - ePub
How Gut and Brain Control Metabolism
- 204 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
How Gut and Brain Control Metabolism
About this book
Obesity is an epidemic problem not limited to Western society, but also in emerging industrial nations with large populations, especially in Asia. The connection between the gut and the brain is probably one of the most promising therapeutic targets for the treatment of obesity and metabolic syndrome. This book brings together reviews on the current understanding of how the gut and brain communicate in the regulation of metabolism. Individual chapters explore novel aspects of this interaction. A comprehensive update on the roles of smell and taste, the gut microbiome, and novel gut-derived neuropeptides in regulating metabolism via the brain is offered. Furthermore, the regulation of insulin sensitivity in the brain is discussed in detail. This overview of current findings is meant to spark new ideas and/or approaches, thus leading to much-needed new medical treatments. Physicians involved in the treatment of metabolic disease and scientists performing research in the fields of nutrition and obesity will find this book to be a valuable addition to their bookshelves.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access How Gut and Brain Control Metabolism by P. J. D. Delhanty,A. J. van der Lely,P.J.D., Delhanty,A.J., van der Lely, Federica Guaraldi,Giovanni Corona,Federica, Guaraldi,Giovanni, Corona in PDF and/or ePUB format, as well as other popular books in Medicine & Nutrition, Dietics & Bariatrics. We have over one million books available in our catalogue for you to explore.
Information
Aspects of Metabolic Interplay between the Brain and the Gut
Delhanty PJD, van der Lely AJ (eds): How Gut and Brain Control Metabolism.
Front Horm Res. Basel, Karger, 2014, vol 42, pp 1-28 (DOI: 10.1159/000358312)
Front Horm Res. Basel, Karger, 2014, vol 42, pp 1-28 (DOI: 10.1159/000358312)
______________________
Hormonal Control of Metabolism by the Hypothalamus-Autonomic Nervous System-Liver Axis
Andries Kalsbeeka, b · Eveline Bruinstroopa · Chun-Xia Yib · Lars Klieverika · Ji Liua · Eric Fliersa
aDepartment of Endocrinology and Metabolism, Academic Medical Center, University of Amsterdam, and bHypothalamic Integration Mechanisms, Netherlands Institute for Neuroscience, Amsterdam, The Netherlands
______________________
Abstract
The hypothalamus has long been appreciated to be fundamental in the control and coordination of homeostatic activity. Historically, this has been viewed in terms of the extensive neuroendocrine control system resulting from processing of hypothalamic signals relayed to the pituitary. Through these actions, endocrine signals are integrated throughout the body, modulating a vast array of physiological processes. Our understanding of the responses to endocrine signals is crucial for the diagnosis and management of many pathological conditions. More recently, the control emanating from the hypothalamus over the autonomic nervous system has been increasingly recognized as a powerful additional modulator of peripheral tissues. However, the neuroendocrine and autonomic control pathways emanating from the hypothalamus are not separate processes. They appear to act as a single integrated regulatory system, far more subtle and complex than when each is viewed in isolation. Consequently, hypothalamic regulation should be viewed as a summation of both neuroendocrine and autonomic influences. The neural regulation is believed to be fine and rapid, whereas the hormonal regulation is more stable and widespread. In this chapter, we will focus on the hypothalamic control of hepatic glucose and lipid metabolism.
© 2014 S. Karger AG, Basel
Dual Control by the Biological Clock
A nice example of this dual control mechanism used by the hypothalamus is provided by the control of the biological clock over daily rhythms in hormone release. The mammalian biological clock resides in the suprachiasmatic nuclei (SCN) located in the anterior hypothalamus. We showed that the SCN uses its projections to both the neuroendocrine and pre-autonomic hypothalamic neurons for generating the daily rhythm in plasma corticosterone concentrations [1]. On the one hand the SCN uses its projections to the subparaventricular nucleus (subPVN) and the dorsomedial hypothalamus (DMH) to modulate the activity of the CRH neurons in the PVN and the subsequent release of ACTH from the pituitary, whereas on the other hand it uses its GABAergic and glutamatergic projections to the pre-autonomic neurons in the PVN to control the sensitivity of the adrenal cortex to the incoming ACTH signal. Another example was recently provided by the work on the circadian regulation of the osmo-regulatory circuit [2].
Our investigations on the circadian control of plasma glucose concentrations also yielded more insight into the hypothalamic control mechanisms of glucose homeostasis. It turned out that the SCN uses its GABAergic and glutamatergic projections to the sympathetic pre-autonomic neurons in the PVN to control hepatic glucose production, and its GABAergic projections to the parasympathetic pre-autonomic neurons in the PVN to control the meal-induced insulin responses from the endocrine pancreas. In addition, these experiments provided evidence for an important role of the hypothalamic orexin, PACAP and VIP systems in the (circadian) control of hepatic glucose production [3].
Neuroanatomical Connections between Hypothalamus and Liver
The brain is a major energy-consuming organ, and it depends almost entirely on glucose as a substrate. It is therefore not surprising that the plasma glucose concentration is tightly controlled by an efficient and complex regulatory neural system. By some this elaborate regulatory system and its safeguard of brain glucose supply, even - if necessary - at the expense of other organ systems, has been phrased as the ‘selfish brain’. The hypothalamus is a key component of this system. Next to the intrinsic hypothalamic control of glucose metabolism by the biological clock it has become clear that the hypothalamus also serves as an important relay center for (humoral) feedback information from the periphery, with important roles for hypothalamic leptin [4] and more recently insulin receptors [5, 6] as striking examples. The hypothalamus consists of different nuclei with distinct neuronal populations. The hypothalamic arcuate nucleus (ARC) is localized at the base of the hypothalamus where the blood-brain barrier is largely absent. Neurons in the ARC express a variety of nutrient and hormone receptors, including leptin, insulin and thyroid hormone receptors. In close proximity to the ARC are the ventromedial hypothalamus (VMH), DMH and lateral hypothalamus (LH), which are also known to be anatomically connected and functionally implicated in energy metabolism [7]. Located centrally in the hypothalamus, the PVN is believed to integrate a variety of signals both from within (ARC, VMH, SCN, LH) and outside the hypothalamus. The PVN integrates this information with signals from other brain regions, and uses several output pathways for the regulation of peripheral metabolism. One of these pathways is the classical neuroendocrine route via the median eminence to the anterior pituitary, which in turn regulates a variety of endocrine glands like the adrenal, gonads and thyroid. Another pathway arising from the PVN relays neural information from its pre-autonomic neurons to the autonomic nervous system (ANS). The neural connection between the brain and the liver consists of both branches of the ANS, i.e. the sympathetic and parasympathetic nervous system. Retrograde tracing studies have shown that sympathetic and parasympathetic connections to the liver originate from different brain regions in the hypothalamus and brainstem [7-9].
Sympathetic Nervous System
All sympathetic input to the liver is relayed via the sympathetic preganglionic cells in the lateral horn of the thoracic spinal cord. These cell bodies lie in the intermediolateral column (IML) and associated cell groups. In the hypothalamus, the pre-autonomic neurons in the PVN and LH send direct projections to the preganglionic neurons in the IML or project indirectly to the IML via brainstem circuits [10]. In the brainstem, central autonomic regions that show direct projections to the preganglionic motoneurons in the IML are the rostroventrolateral medulla, A5 region and the parapyramidal region (which can be separated into ventromedial medulla and raphe nucleus), which send direct projections to the IML. The IML in the spinal cord is connected via splanchnic nerves to the celiac ganglion innervating the liver. Sympathetic hepatic nerves innervate the liver through nerve bundles that accompany the large vessels in the liver hilus from where they penetrate to different extents into the acinus. Large species differences exist in the extent of sympathetic innervation of liver parenchyma [11] (fig. 1).
Sympathetic hepatic nerves can modulate hepatocyte function by direct action of their neurotransmitter noradrenaline on the α- and β-adrenergic receptors. Besides noradrenaline, sympathetic hepatic nerve endings may also release neuropeptides, such as NPY and galanin.
Parasympathetic Nervous System
The efferent parasympathetic autonomic signal is conveyed via preganglionic cells in the dorsal motonucleus of the vagus (DMV) in the brainstem. Tracing studies combining a sympathetic denervation of the liver with injection of a tracer confirm that many of the above-mentioned central autonomic nuclei (PVN, LH, A5, parapyramidal area) also contribute to the control of the parasympathetic function, although from separate neuronal populations, thus indicating a functional specialization [9]. The DMV via the vagal nerve directly connects to ganglion cells, without involvement of the spinal cord (fig. 2).
The right posterior subdiaphragmatic vagal nerve branches into the left and right hepatic branch proper and the ganglion cells concerned are located close to the liver. Postganglionic parasympathetic nerves mainly use acetylcholine as their neurotransmitter, although peptides (such as cholecystokinin) are also involved. Acetylcholine acts on two types of receptors, the muscarinic and nicotinic cholinergic receptors.
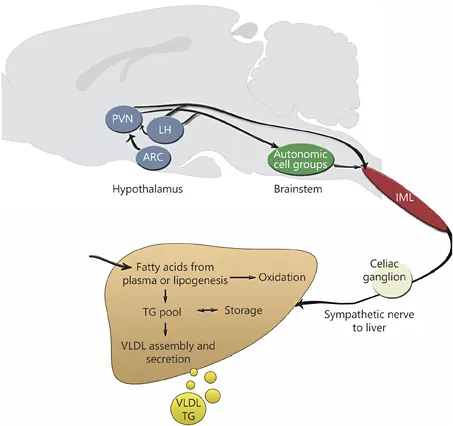
Fig. 1. Graphical representation of the efferent sympathetic connections between the brain and liver and a schematic representation of hepatic lipid metabolism.
Hypothalamic Control of Liver Metabolism via the Autonomic Nervous System
It is widely assumed that metabolic changes observed during metabolic and hormonal disorders such as type 2 diabetes, but also for instance thyrotoxicosis, Cushing’s syndrome and menopause, are mediated via direct actions of insulin, thyroid hormone, cortisol and estrogen, respectively, on hormone receptors in peripheral organs such as the liver, muscle and adipose tissue. However, as will be shown in this chapter, the metabolic changes induced by insulin, thyroid hormone, glucocorticoids and estrogen are also partly mediated via the hypothalamus and the ANS. This concept is nicely illustrated by insulin, which is known to lower plasma glucose by facilitating glucose uptake in muscle and adipose tissue, as well as by inhibiting endogenous glucose production (EGP) in the liver. The latter effect is mediated by insulin’s actions on several aspects of glucose metabolism including glycogenolysis and gluconeogenesis in the hepatocyte. At the turn of the century, however, it became clear that in rodents, EGP is inhibited by 40% upon low dose intracerebroventricular (ICV) infusion of insulin, and this occurs independently of circulating insulin concentrations [5, 6].
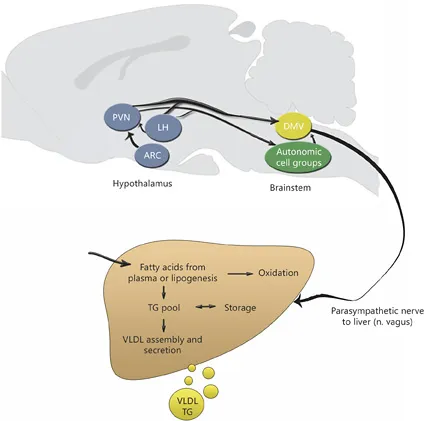
Fig. 2. Graphical representation of the efferent parasympathetic connections between the brain and liver and a schematic representation of hepatic lipid metabolism.
The pathway involved in this central effect of insulin on hepatic glucose metabolism has been partly unravelled, and involves insulin receptors in the ARC, hypothal...
Table of contents
- Cover Page
- Front Matter
- Aspects of Metabolic Interplay between the Brain and the Gut
- Smell and Taste
- The Role of Neuropeptides in Metabolism
- Author Index
- Subject Index