
eBook - ePub
Adult Stem Cells in Aging, Diseases and Cancer
- 88 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Adult Stem Cells in Aging, Diseases and Cancer
About this book
The functionality of adult tissue stem cells from various organ systems declines during aging. This publication summarizes novel molecular mechanisms responsible for the development of aging-associated deficiencies as discussed by leading experts during the 5th Else Kröner-Fresenius Symposium. It is the first book that explicitly focuses on molecular mechanisms of stem cell aging and its consequences for disease and cancer development including both cell-intrinsic mechanisms as well as aging-induced alterations in the stem cell niche and the systemic environment. Cutting-edge information on stem cells, aging, cancer, and disease make this publication of special interest to basic researchers in the respective fields. Further, it is also intended for medical doctors in the fields of geriatrics, internal medicine, and cancer as it provides a novel understanding of the evolution of tissue dysfunction, diseases and cancer as a consequence of aging.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Adult Stem Cells in Aging, Diseases and Cancer by K. L. Rudolph,K.L., Rudolph, S. Pahernik,S., Pahernik in PDF and/or ePUB format, as well as other popular books in Biological Sciences & Alternative & Complementary Medicine. We have over one million books available in our catalogue for you to explore.
Information
Rudolph KL (ed): Adult Stem Cells in Aging, Diseases and Cancer.
Else Kröner-Fresenius Symp. Basel, Karger, 2015, vol 5, pp 40-59 (DOI: 10.1159/000366570)
Else Kröner-Fresenius Symp. Basel, Karger, 2015, vol 5, pp 40-59 (DOI: 10.1159/000366570)
______________________
Mechanism of Functional Alterations in Hematopoietic Stem Cell Aging
Yohei Morita
Leibniz Institute for Age Research, Fritz Lipmann Institute, Jena, Germany
______________________
Abstract
Aging leads to functional decline of hematopoietic stem cells (HSCs), including alterations of self-renewal and differentiation, which is thought to contribute to age-related hematopoietic disorders, defects in immune functions, and an increase in hematopoietic malignancies. Different mechanisms could influence the functional decline or the malignant transformation of HSCs during aging. (a) The maintenance of HSC quiescence: low cell cycle activity is a characteristic sign of HSCs, and this may prevent the accumulation of replication-induced DNA damage and epigenetic alteration of the DNA. However, quiescent HSCs are also more vulnerable to accumulate DNA damage as homologous recombination-dependent DNA repair is only active in cycling cells. (b) DNA damage checkpoints: apoptosis and senescence could prevent the survival of damaged and/or mutant HSCs but may simultaneously provoke aging by diminishing the self-renewal of aging HSCs. Clarification of molecular changes in aging HSCs will not only help our understanding of age-related bone marrow failure and immunosenescence, but will also provide insights into the molecular basis for increased hematopoietic malignancies during aging. An understanding of these age-dependent processes could ultimately provide a rational basis for the development of new treatment and prevention strategies for age-associated bone marrow failure, immune dysfunction, and leukemia development.
© 2014 S. Karger AG, Basel
Characteristics of Hematopoietic Stem Cells
Hematopoietic stem cells (HSCs) are the best-characterized somatic stem cells in the mammalian system [1] ensuring the maintenance and the development of all blood components, providing about 1011-1012 new blood cells daily in humans [2]. HSCs can self-renew and produce progenitor cells differentiating into mature cells, thus fitting the concept of hierarchal organization. A hierarchical structure in the hematopoietic system is experimentally documented in a way that the most primitive stem cells reside at the apex of the hierarchy capable of both, self-renewal and generation of progenitor cells differentiating into a variety of mature cell types (fig. 1) [3].
The most primitive HSCs are long-term HSCs (LT-HSCs) that are characterized by a broad range of surface markers [4-10]. LT-HSCs are negative for lineage markers, CD48, CD244, CD34, Flt3, and N-cadherin, but positive for Tie2, Endoglin, Thy1 (preferably expressed on lower levels), Sca-1, c-Kit, CD38 and CD150. In experimental setups, LT-HSCs are mainly sorted as CD150+CD34lo/- Flt3-KSL cells (low or negative of CD34, Flt3-negative, lineage-negative, CD150-positive, Sca1-positive and c-Kit-positive). This approach allows enrichment for LT-HSCs - when transplanted as single cells, 30-50% of the purified cells have the potential for long-term multi-lineage reconstitution in lethally irradiated mice by differentiating in all blood cell types [9]. The true percentage of LT-HSCs in this population might be underestimated due to experimental error in single cell transplants. In contrast to LT-HSCs, short-term HSCs (ST-HSCs) have already initiated differentiation and lack long-term reconstitution potential [11-15]. ST-HSCs differentiate into multipotent progenitor cells (MPPs) that lack self-renewal potential [8] but can differentiate into common myeloid and lymphoid progenitors (CMPs [16] and CLPs [17]), which further differentiate into functional blood cells.
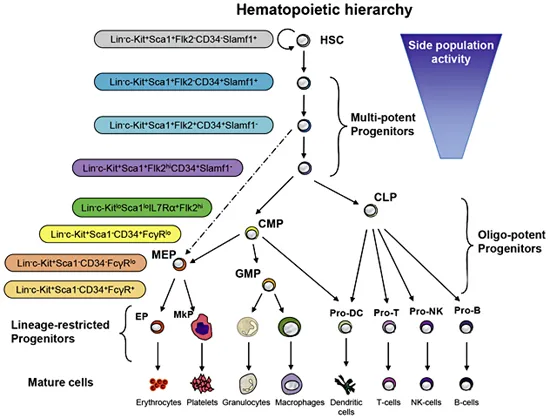
Fig. 1. Overview of the hematopoietic hierarchy and cell surface markers characterizing the different progenitor cells [18]. HSCs have self-renewal activity and first differentiate into multipotent progenitors that produce CMPs and CLPs. CMPs can differentiate into granulocyte/macrophage progenitors (GMPs) and megakaryocyte/erythrocyte progenitors (MEPs). CMPs and CLPs are responsible for myelopoiesis and lymphopoiesis, respectively.
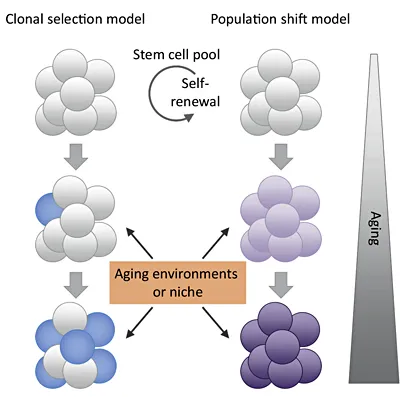
Fig. 2. Current models of stem cell aging [26]. The ‘clonal selection model of stem cell aging’ suggests that certain stem cell clones that bear distinct functional potentials are able to outcompete other types of clones during aging. Over time, such clones will come to predominate the stem cell pool, whereas others become diminished. In contrast, the ‘population shift model of stem cell aging’ suggests that the functional potential of all the cells in the stem cell population is equivalent at any stage of ontogeny, but the potential gradually and coordinately changes over time. These models are not mutually exclusive and both may be instructed by cell-intrinsic and non-cell autonomous cues originating from the aging micro- or systemic environment.
Functional Alteration of Hematopoietic Stem Cells during Aging
Previous investigations indicated that clonal selection is contributing to the process of HSC aging (fig. 2). Work from Christa Muller-Sieburg first showed that there are three different populations of HSCs: (a) balanced HSCs (Bala), (b) lymphoid-biased HSCs (Ly-bi), and (c) myeloid-biased HSCs (My-bi). All types of HSC produce myeloid and lymphoid cells in blood; however, Ly-bi HSCs predominantly generate lymphoid cells, and My-bi HSCs predominantly generate myeloid cells compared with Bala HSCs producing both lineages. It was shown that the different HSC subtypes show no obvious aging phenotype, but the relative proportion of these individual subpopulations changes during aging. This population shift leads to differences in the reconstitution capacities of the total pool of HSCs during aging. The My-bi HSCs accumulate with aging, whereas the other 2 subpopulations of HSCs decrease, resulting in an age-dependent skewing of hematolymphopoiesis with a decrease in lymphopoiesis and an increase in myelopoiesis. This phenotype is mouse strain-independent only showing minor inter-strain variations, e.g. a lower percentage of My-bi HSCs in DBA mice compared to C57BL/6 mice. In summary, the selective survival of My-bi HSCs appears to be an important contributor to impaired lymphocyte production in aging mice [19, 20]. Similar findings were recapitulated by other laboratories [21]. Recent studies showed that the CD150 marker can distinguish myeloid-biased from lymphoid-biased HSCs. Transplantation of CD150highCD34lo/-KSL cells results in myeloid-dominant reconstitution, whereas CD150lowCD34lo/-KSL cells show a balanced lymph-myeloid reconstitution [22, 23]. CD150highCD34lo/-KSL cells have a molecular profile that appears myelo-erythroid primed, while CD150lowCD34lo/-KSL cells are more lymphoid primed. The greater self-renewal potential of myeloid-biased CD150highCD34lo/-KSL cells resulted in their expansion in the primitive stem cell pool during aging. Together, these studies support the model of clonal selection of subpopulations of HSCs during aging (fig. 2). However, the model remains under debate; an important question is to delineate the plasticity of different subpopulations of HSCs and whether one subpopulation can give rise to another subpopulation of HSCs.
Due to the phenotypic and quantitative changes of the stem cell pool in the hematopoietic system during aging, it is of great interest to estimate the stem cell pool size. Dr. de Haan introduced a retroviral cellular barcoding tool to track the clonality of HSCs [24]. Retroviral bar-coded vector is used for transduction of HSCs in vitro, assuming that only one virus particle infects one HSC. After transplantation of single-infected HSCs, deep sequencing analysis can be performed on the different output populations (B cells, T cells and granulocytes) at different time points. Using a variety of barcodes, it can be estimated to what quantity and quality certain HSC subclones contribute to reconstitution. This system provides an intelligent tool to track HSCs during reconstitution and estimate their cell destiny. Using this system, Dr. de Haan showed that contribution of HSCs to mature blood cells in peripheral blood stabilizes at 12 weeks after transplantation. The clonal composition within each lineage after the stabilization showed good correlation, but the output of individual HSCs in HSC population of bone marrow (BM), and granulocytes, T cells and B cells of peripheral blood was quite different. To investigate differences of clonality between young and old HSCs, they transplanted young and old mixed HSCs (1:2 ratio) transduced with barcoded virus into lethally irradiated mice. They showed that old HSCs cannot efficiently produce mature cells in peripheral blood compared to young HSCs, although old HSCs can stay in the HSC compartment of BM, indicating diminished differentiation activity of old HSCs. They also showed that the clonality of old HSCs is always higher than that of young HSCs, whereas clone size of old HSCs is always smaller in the recipients. These data may imply that homing efficiency of old HSCs is equivalent to young HSCs and that old HSCs need to be proliferated more to maintain hematopoiesis because of the diminished repopulating activity.
Dr. Rodewald investigated the contribution of HSCs to hematopoiesis in steady state using a different cell tracing system. To exclude possible effects on hematopoiesis by viral insertion or transplantation, Dr. Katrin Busch in the Rodewald laboratory established a mouse line enabling in vivo lineage tracing. The system uses tamoxifen-inducible YFP expression in HSCs. In response to tamoxifen injection, a small number of functional HSCs were labeled by YFP, which was confirmed by measuring repopulating activity of single YFP+ HSCs after transplantation. The labeled HSCs differentiate into progenitors and eventually produce mature cells with YFP labeling. They analyzed the relationship between HSC and progenitors by YFP labeling frequency in each hematopoietic population from approximately 100 mice, and found strong correlations between LT-HSC and ST-HSC, ST-HSC and MPP, and MPP and CMP. In contrast, no correlation between MPP and CLP was found. These data suggest that conventional differentiation pathway from LT-HSCs to granulocytes is solid, but that CLPs might be not direc...
Table of contents
- Cover Page
- Front Matter
- Introduction
- Speakers at the Symposium
- Stem Cells in Adult Intestine
- Molecular Mechanisms of Muscle Stem Cell Aging
- Mechanism of Functional Alterations in Hematopoietic Stem Cell Aging
- The Microenvironment, Aging and Disease
- Sestrins in Aging and Metabolism
- Telomeres and Stem Cell Aging
- DNA Damage and Checkpoint Responses in Adult Stem Cells
- Thomas Hell Plays ‘Vertigo’ by György Ligeti
- Author Index
- Subject Index