
eBook - ePub
Advanced Therapies in Pediatric Endocrinology and Diabetology
- 166 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Advanced Therapies in Pediatric Endocrinology and Diabetology
About this book
Dealing with current hot topics, this book aims to provide an update on advances in the treatment of endocrine disorders. The chapters cover innovative therapeutic approaches to type 1 diabetes, such as artificial pancreas and islet transplantation. Additionally, novel pharmacological interventions for patients with obesity and the metabolic syndrome, as well as with adrenal and gonadal disorders are provided. Furthermore, recent developments in growth-promoting therapies, such as the C-type natriuretic peptide analog to treat achondroplasia, as well as antioxidant and gene replacement therapies in patients with adrenoleukodystrophy, are described. Worldwide renowned experts share their experience and cutting edge research in Advanced Therapies in Pediatric Endocrinology and Diabetology, which makes it a useful and interesting resource for every pediatric endocrinologist, not only those active in clinical research, but also those involved in the management of children with endocrine diseases in daily practice.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Advanced Therapies in Pediatric Endocrinology and Diabetology by M. Cappa,S. Cianfarani,L. Ghizzoni,S. Loche,M. Maghnie,M., Cappa,S., Cianfarani,L., Ghizzoni,S., Loche,M., Maghnie, M. Cappa, S. Cianfarani, L. Ghizzoni, S. Loche, M. Maghnie in PDF and/or ePUB format, as well as other popular books in Medicine & Endocrinology & Metabolism. We have over one million books available in our catalogue for you to explore.
Information
Cappa M, Cianfarani S, Ghizzoni L, Loche S, Maghnie M (eds): Advanced Therapies in Pediatric Endocrinology and Diabetology. Endocr Dev. Basel, Karger, 2016, vol 30, pp 1-13 (DOI: 10.1159/000439321)
______________________
Toward Automation of Insulin Delivery - Management Solutions for Type 1 Diabetes
Revital Nimria · Moshe Phillipa, b
aThe Jesse Z and Sara Lea Shafer Institute for Endocrinology and Diabetes, National Center for Childhood Diabetes, Schneider Children's Medical Center of Israel, Petah Tikva, and bSackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
______________________
Abstract
In the past decade, the field of type 1 diabetes was characterized by the efforts to integrate technology into the daily management of diabetes. Automated insulin delivery systems have emerged followed by the improvements in technology of pumps and sensors and automated close-loop systems that were developed around the world for overnight as well as for day and night use. Initially, these closed-loop systems were tested clinically in research centers, then at diabetes camps or hotels, and recently at patients’ homes. The systems were tested in a wide range of populations of patients with type 1 diabetes: children, adolescents, adults, newly diagnosed, well and suboptimally controlled patients, the critically ill and pregnant women. The extensive clinical evaluation found these close-loop systems to be safe and efficient in controlling blood glucose levels. Now is the time to take these systems from research to industry and to get a regulatory approval of convenient devices for the use at home. Automated insulin delivery systems have the potential to change the way diabetes is treated and managed for the benefit of patients. This chapter summarizes the recent advances in this field.
© 2016 S. Karger AG, Basel
A century passed since the discovery of insulin in Toronto and two decades since the results of the Diabetes Control and Complications Trial were published and the world acknowledgment that good glycemic control is mandatory to reduce or postpone diabetes-related complications. In the past years, new technologies have emerged for better blood glucose monitoring, insulin delivery devices, bolus calculators, insulin analogs and more. However, patients and caregivers still struggle to manage diabetes, and the majority of patients with type 1 diabetes around the world do not achieve the desired glycemic control. The data from the pediatric T1D Exchange clinic registry, which includes 13,316 patients, showed that only 32 and 25% of the patients met the age-specific hemoglobin A1c target levels recommended by the American Diabetes Association and International Society for Pediatric and Adolescent Diabetes, respectively [1]. Maintaining tight glycemic control is associated with an increased risk of hypoglycemia and entails great effort, tension and anxiety that patients with diabetes face at all times. Automated decision support systems and ultimately fully automated management solutions for patients with type 1 diabetes are needed.
The automation of insulin delivery and closed-loop systems have gained the focus of research in diabetes in recent years [2]. The basic principle of these systems is an automated insulin delivery derived by control algorithm which calculates the amount of insulin needed to keep the blood glucose levels within a desired range based on real-time glucose levels measured by a glucose sensor and then delivered automatically to the patient via an insulin pump.
The foundation of the closed-loop approach was laid over 45 years ago by Kadish [3] in 1963. The pioneer system had a real-time continuous glucose monitor and two delivery arms: one for insulin and a counterregulatory arm for glucose or glucagon. However, the system worked on a simple feedback controller and lacked meaningful computer powering. A decade passed until computer control devices were incorporated in the system, creating the ‘true artificial pancreas’ in 1974 by Albisser et al. [4] in Toronto and Pfeiffer et al. [5] in Germany. The commercial device named Biostator was produced in the 1970s. However, it was a huge and bulky bedside device which needed constant supervision, and hence was abandoned from clinical use. The field of closed-loop insulin delivery was then halted for about two decades. With the remarkable technological progress, including miniaturizations of devices, pumps, interstitial sensors, powered computers and wireless communications, interest in the closed-loop insulin delivery was revived.
Several groups around the world have developed and tested the system in a growing number of clinical studies. These systems were found to be safe, improved metabolic control and solved frequent problems in diabetology. Most importantly, these systems were shown to improve the quality of life of patients and to relieve some of the heavy burden of managing diabetes. The next step is to transfer the software from the clinical research into an industrial product. Based on the results of the clinical trials, it is clear that the time is right for regulatory approval and commercialization of such systems.
Types of Closed-Loop Systems
A wide range of closed-loop systems have been developed and tested by different research groups around the world. There are two main approaches to automatically control blood glucose excursions in subjects with diabetes. The first and most common approach is the single-hormone delivery of insulin to lower blood glucose. The second is a dual-hormonal delivery approach that adds glucagon as a counterregulatory hormone together with insulin. Closed-loop systems may also differ in the route for hormone delivery and glucose sensing, and in the type of the control algorithm used.
There are several combinations to monitor glucose levels and deliver insulin in a closed-loop system. The most common closed-loop systems are based on subcutaneous glucose sensing and insulin delivery. This is the most convenient, noninvasive way to close the loop since it is the regular route for insulin delivery and glucose monitoring used nowadays by patients. This kind of closed-loop systems can be more readily approved by regulatory bodies for clinical use since they utilize already available insulin pumps and glucose sensors.
Nevertheless, the use of subcutaneous insulin delivery has several limitations. These include a delay in subcutaneous insulin absorption and action and lack of reproducibility which lead to postprandial hyperglycemia and late hypoglycemia, especially when used to cover meals. These limitations turn the mission of closing the loop during meals into a challenging one. Therefore, another option for closed-loop operation is to sense glucose in the subcutaneous tissue and to deliver insulin intraperitoneally or intravenously in order to improve the insulin action profile and to better mimic the physiological way of insulin delivery.
Renard et al. [6] used an intraperitoneally implanted pump and subcutaneous insulin delivery during closed-loop control for 2 days in 8 adults. However, this method did not overcome the postprandial glucose excursion and patients still needed to give a preprandial insulin bolus. The disadvantages of this approach include the need to implant a pump and to periodically refill it with insulin in the hospital, risk of site infection and increased risk to develop insulin antibodies.
Different types of control algorithms were developed over the past years. These sophisticated calculators utilize transmitted glucose levels for analysis of the needed insulin treatment dose. In addition, several systems set individualized target glucose levels and take the diversity in insulin sensitivity in different patients and within the same patient into consideration. Thus, a closed-loop system needs to be personalized and to have an active and dynamic system that can change treatment periodically according to real-time learning of the unpredictable behavior of glucose-insulin dynamics [7].
The first control algorithm that was used clinically was the proportional-integral-derivative algorithm by Steil et al. [8]. Their control algorithm had three components that all together dictated the amount of the total insulin treatment dose: the proportional component calculated the deviation of the blood glucose level from the target glucose level, the integral component calculated the area under the curve between the blood and target glucose level, and the third derivative component took into account the rate of glucose change into account. There is another way to calculate the needed amount of insulin which is by using of the model predictive controller (MPC). This control algorithm - which is mostly used and studied - predicts the upcoming blood glucose levels based on physiological models of human glucose metabolism. The algorithm adjusts the insulin treatment in order to bring the predicted glucose levels into the target range. These two types of algorithms use mathematical models to link glucose excursions to insulin demands.
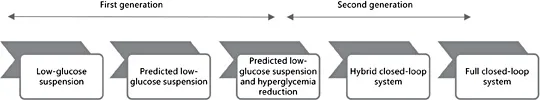
Fig. 1. Artificial pancreas step/generation diagram - the development pathway (with permission from JDRF).
An alternative way to calculate insulin delivery is to use the regular set of rules that patients and caregivers use to treat and manage diabetes in daily life. This method of insulin delivery is a unique clinical-driven technology based on a fuzzy logic control algorithm [9].
The Pathway Towards Closed-Loop Systems
Closed-loop systems were recognized by several important organizations as a potential management solution for patients with type 1 diabetes. In 2006, the Juvenile Diabetes Research Foundation (JDRF) and in 2010 the European Union established the artificial pancreas consortiums (JDRF Artificial Pancreas Project and AP@Home). These organizations and others have been making great efforts to promote the development of a commercially available artificial pancreas.
The JDRF strategic for stepwise implementation of automated insulin delivery is presented in the - ‘artificial pancreas step/generation’ diagram (fig. 1). This strategy includes three generations of automated insulin delivery systems. The first generation includes the devices that shut off insulin delivery to prevent episodes of hypoglycemia. Hypoglycemia remains a major barrier for glycemic control even in the era of advanced technologies. The second generation consists of closed-loop systems that are able to increase the time within the target range and reduce the rate of hypoglycemia. This category includes two closed-loop systems: one system that allows a certain degree of patient involvement at meal or physical activity announcement (hybrid closed-loop system) and the other is the ultimate desired system with no need from the patient to intervene (full closed-loop system). As delineated by the JDRF, the third generation includes the future device for closed-loop systems that will incorporate other hormones besides insulin.
Low/Predicted Glucose Suspension
The first generation of automated insulin delivery devices is featured with the aim to reduce hypoglycemia. These technologies include the low and predicted glucose suspension features incorporated in the sensor-augmented pump therapy.
The first approved automated insulin delivery system is the threshold-suspend feature of the sensor-augmented insulin pumps (MiniMed 530G System; Medtronic Diabetes, Northridge, Calif., USA). This feature is designed to automatically suspend insulin delivery for up to 2 h when the glucose level reaches a predefined low sensor glucose threshold [10].
The safety and efficacy of this system in reducing the risk of nocturnal hypoglycemia was tested in several clinical studies by the ASPIRE In-Home study group. Two large-scale studies point out the efficacy of this system in reducing hypoglycemia with a mild but nonsignificant increase in hyperglycemia [11, 12].
Bergenstal et al. [11] studied 247 subjects with type 1 diabetes prone to nocturnal hypoglycemia treated with insulin pump therapy. Subjects were randomly assigned to participate in a 3-month study with or without the threshold-suspend feature activated in their pumps. The glucose threshold fo...
Table of contents
- Cover Page
- Front Matter
- Toward Automation of Insulin Delivery - Management Solutions for Type 1 Diabetes
- Islet Transplantation in Pediatric Patients: Current Indications and Future Perspectives
- Glucagon-Like Peptide-1 Receptor Agonist Treatment for Pediatric Obesity
- Docosahexaenoic Acid and Its Role in G-Protein-Coupled Receptor 120 Activation in Children Affected by Nonalcoholic Fatty Liver Disease
- Noninvasive Prenatal Diagnosis of Congenital Adrenal Hyperplasia
- Recent Advances in Hydrocortisone Replacement Treatment
- Experience with the Histrelin Implant in Pediatric Patients
- Different Medications for Hypogonadotropic Hypogonadism
- Long-Acting Growth Hormone: An Update
- C-Type Natriuretic Peptide Analog as Therapy for Achondroplasia
- Therapeutic Neuroendocrine Agonist and Antagonist Analogs of Hypothalamic Neuropeptides as Modulators of the Hypothalamic-Pituitary-Gonadal Axis
- Aromatase Inhibitors in the Treatment of Short Stature
- Gene Therapy for Rare Central Nervous System Diseases Comes to Age
- Novel Therapeutic Targets and Drug Candidates for Modifying Disease Progression in Adrenoleukodystrophy
- Author Index
- Subject Index