
eBook - ePub
OCT Angiography in Retinal and Macular Diseases
- 184 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
OCT Angiography in Retinal and Macular Diseases
About this book
In only a short period of time, the innovative procedure of OCT angiography has become an essential macula imaging technique. Now that it is routinely used in clinical practice, the investigation of retinal and choroidal circulation is non-invasive, which significantly changes the professional's approach to patients. In this volume, retina specialists and renowned experts share their experience with OCT angiography. They have included numerous color images and presented current ideas to form a base for further research and discussion. This book provides retina specialists, ophthalmologists, and researchers with a first glance at original research and clinical reports on this new methodology.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access OCT Angiography in Retinal and Macular Diseases by F. Bandello,E. H. Souied,G. Querques,F., Bandello,E.H., Souied,G., Querques, F. Bandello,F., Bandello in PDF and/or ePUB format, as well as other popular books in Medicine & Genetics in Medicine. We have over one million books available in our catalogue for you to explore.
Information
Bandello F, Souied EH, Querques G (eds): OCT Angiography in Retinal and Macular Diseases.
Dev Ophthalmol. Basel, Karger, 2016, vol 56, pp 45-51 (DOI: 10.1159/000442776)
Dev Ophthalmol. Basel, Karger, 2016, vol 56, pp 45-51 (DOI: 10.1159/000442776)
______________________
Optical Coherence Tomography Angiography of Type 1 Neovascularization in Age-Related Macular Degeneration
Nicholas A. Iafea · Nopasak Phasukkijwatanaa · David Sarrafa, b
aStein Eye Institute, David Geffen School of Medicine at University of California Los Angeles, and bGreater Los Angeles VA Healthcare Center, Los Angeles, Calif., USA
______________________
Abstract
Age-related macular degeneration continues to be the leading cause of severe central vision loss in older adults of European descent. Optical coherence tomography angiography (OCT-A) enables more accurate identification of type 1 neovascularization in age-related macular degeneration than traditional fluorescein and indocyanine green angiographies. In addition, OCT-A facilitates the morphological classification of type 1 lesions, including features characteristic of early, mature, and fibrotic lesions. Vessel complex analysis, including lesion area and capillary density quantification, can also be readily measured and monitored over time. Performing this analysis following anti-vascular endothelial growth factor therapy may lead to a better understanding of the efficacies and responses to such treatments. Although some limitations currently exist, OCT-A is a promising imaging modality that could prove to have profound implications if incorporated into regular clinical practice.
© 2016 S. Karger AG, Basel
Age-related macular degeneration (AMD) continues to be the leading cause of blindness among individuals older than 50 years of age in the developed world [1]. Neovascular AMD is the etiology for severe vision loss in 90% of AMD cases. Three lesion subtypes, best classified on the basis of spectral domain optical coherence tomography (OCT), comprise the neovascular form of this disease [2]. Type 1 neovascularization originates from the choriocapillaris and is localized under the retinal pigment epithelium. Type 2 neovascularization also originates from the choriocapillaris but extends through the retinal pigment epithelium and is localized in the subretinal compartment. Type 3 neovascularization originates from the deep retinal capillary plexus [2-4] and is located in the outer retina. Type 1 neovascularization is the most common neovascular subtype of AMD [5].
Recent advancements in OCT angiography (OCT-A) have provided retinologists with a window to directly identify the morphologies of neovascular subtypes in AMD. OCT-A enables more accurate identification of type 1 lesions compared to traditional fluorescein angiography (FA). While FA can identify the superficial retinal capillary plexus, this imaging modality poorly visualizes the deep retinal capillary plexus and the choroid. Pigment epithelial detachment (PED) may demonstrate pooling or stippled fluorescence with FA, but the identification of the causative neovascular complex is very challenging and only minimally improved with indocyanine green angiography. Conversely, OCT-A utilizes amplitude or phase decorrelation technology with high-frequency and dense volumetric scanning to detect red blood cell movement and to visualize blood vessels at various depth-resolved levels of the retina and choroid [3]. As opposed to FA and indocyanine green angiography, in which the presence of an occult choroidal neovascular membrane is inferred by the presence of pooling within a PED and/or the identification of a hot spot, OCT-A reveals the vessels themselves and enables one to more accurately identify and evaluate the morphology of the neovascular complex.
OCT-A of type 1 neovascularization has lead to a detailed assessment of the microvascular morphologies of these vessel complexes, which are typically hidden under a PED. Numerous studies have identified the different morphologies of these neovascular lesions and have applied varying descriptive terms to label these structures, which are best visualized with OCT-A. These labels include ‘umbrella vessels’ , ‘seafan and medusa vessels’ , ‘tangled network pattern’ , and ‘pruned vascular and blossoming tree’ [3,6,7]. This complicated and indistinct nomenclature has caused confusion in the retina community, and a simpler classification system will certainly evolve that may reflect the chronicity of type 1 neovascularization as identified with OCT-A.
Though the precise precipitating stimulus for angiogenesis in AMD remains to be elucidated, the development of neovascular complexes has been shown to be highly dependent on the presence of vascular endothelial growth factor (VEGF) [8-10]. Hypoperfusion or alteration of the choriocapillaris is often noted in OCT-A in association with type 1 complexes [3,11-13] and is likely one cause of localized increases in VEGF production. The newly established VEGF gradient stimulates the propagation of vascular endothelial cells to form new capillaries [8]. When imaged with OCT-A in this early or acute phase, the neovascularization has the appearance of a tangled web of fine vessels (fig. 1) [8,14]. Muakkassa et al. [14] performed OCT-A on treatment-naïve eyes with type 1 neovascularization, and the lesions were typically small (less than 1 mm2) and comprised of a round tuft of small-caliber capillaries without dilated core feeder vessels.
Chronic type 1 lesions have been noted to demonstrate a distinctly different morphology. In the largest study to date using OCT-A to describe chronic type 1 lesions previously treated with multiple intravitreal anti-VEGF injections, Kuehlewein and associates [3] analyzed 33 eyes with AMD and PED associated with type 1 lesions that were large and mature; these averaged 5.79 mm2 in area. Of note, 75% of the cases showed a highly organized vascular complex with vessels branching from a core trunk (fig. 2) and multiple large, dilated feeder vessels (fig. 3). The existence of well-perfused feeder vessels in chronically treated type 1 lesions has also been identified by Coscas and associates [15]. It has been hypothesized that the feeder vessels and central trunk are more resistant to anti-VEGF therapy because their endothelial cells are protected by overlying pericytes, whereas the finer branching vessels contain unprotected endothelial cells, rendering them more responsive to continued anti-VEGF therapy [16,17]. In his seminal paper, Spaide [8] highlights the distinction between angiogenesis and arteriogenesis and proposes a theory of vascular abnormalization to describe the altered morphology of chronically treated type 1 neovascular complexes. Spaide theorizes that the process of closing smaller pericyte-poor vessels within a neovascular complex in response to anti-VEGF leads to increased vascular resistance within the lesion. The remaining pericyte-rich vessels subsequently experience higher flow and higher intraluminal pressure, thereby creating a stimulus for arteriogenesis and increased vessel size. A cycle of regrowth and pruning of the immature, pericyte-poor vessels at the leading edge of the type 1 complex takes place in response to anti-VEGF therapy, while the mature, dilated pericyte-rich core vessels progressively enlarge. This cycling has been shown to carry a risk of evolution toward subretinal fibrosis [18,19].
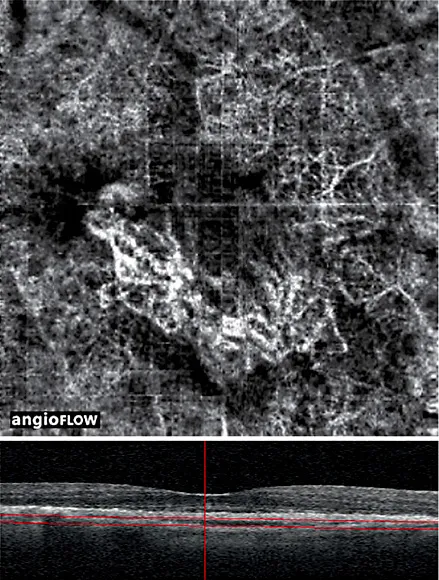
Fig. 1. An 86-year-old patient with treatment-naïve type 1 neovascularization in the right eye. (Top) A 3 mm × 3 mm motion-corrected optical coherence tomography (OCT) angiogram imaged on Avanti RTVue OCTA device showing well-circumscribed choroidal neovascularization with a tangled web of vessels. A quilting artifact due to motion is present. (Bottom) Corresponding spectral domain OCT B-scan with segmentation lines. The authors would like to acknowledge and credit Dr. Nadia Waheed and Dr. Emily Cole for providing these images.
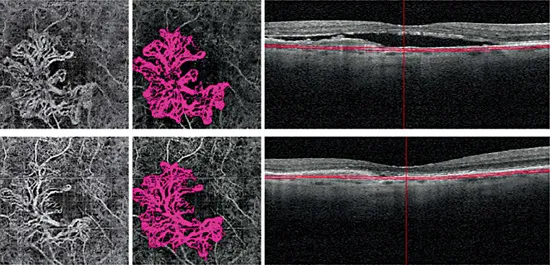
Fig. 2. A 77-year-old male patient with type 1 neovascularization in the right eye, with a visual acuity of 20/40. The patient’s status following 6 aflibercept injections and 7 ranibizumab injections is shown. (Top left) 3 mm x 3 mm OCT angiography (OCT-A) en face projection image of a mature type 1 complex with large feeder vessels and multiple dilated core vessels identified. (Top middle) Corresponding color-coded vessel complex for density analysis. (Top right) OCT B-scan image showing slab segmentation through the pigment epithelial detachment. (Bottom row) Follow-up OCT-A 8 weeks later (interim treatment: 2 aflibercept injections). (Bottom left) 3 mm x 3 mm OCT-A en face projection image of the type 1 complex. (Bottom middle) Corresponding color-coded complex for vessel density analysis. (Bottom right) OCT B-scan image showing slab segmentation. Note that the many large, dilated vessels of this mature type 1 complex are unaffected by additional anti-vascular endothelial growth factor therapy, although the finer vascular plexus may show some attenuation. The lesion area was 3.08 mm2 at baseline and 3.08 mm2 at follow-up; the vessel density was 47% at baseline and 43% at follow-up.
OCT-A has also been employed to study the late fibrotic stage of type 1 neovascularization in AMD. Miere and associates [7] analyzed 49 eyes diagnosed with subretinal fibrosis complicating neovascular AMD, 39 of which were either type 1 or combined type 1 and type 2 lesions. OCT-A demonstrated blood flow related to a persistent neovascular complex within the fibrotic scar in 46 of the 49 eyes [7]. Analysis of these complexes revealed large, dilated vessels with or without vascular loops and interlacing networks, but they typically consisted of only large, mature vessels without an associated fine dense capillary plexus. Most fibrotic lesions also had large flow void areas of the choriocapillaris or dark halos.
Additionally, OCT-A has been utilized to characterize the response of type 1 neovascularization to antiangiogenic therapy. Muakkassa and associates [14] studied six patients with treatment-naïve choroidal neovascularization (CNV), four of which had type 1 lesions. Eyes were scanned before anti-VEGF treatment and at follow-up visits in order to assess the area of each neovascular lesion and its greatest linear dimension (GLD). Follow-up images taken 2-9.5 weeks after initial injection revealed a 29.8% average decrease in CNV area and a 23.6% decrease in its GLD [14]. All patients also experienced improvement or stabilization of...
Table of contents
- Cover Page
- Front Matter
- Heidelberg Spectralis Optical Coherence Tomography Angiography: Technical Aspects
- Optical Coherence Tomography Angiography Using the Optovue Device
- Swept-Source Optical Coherence Tomography Angio™ (Topcon Corp, Japan): Technology Review
- ZEISS Angioplex™ Spectral Domain Optical Coherence Tomography Angiography: Technical Aspects
- Image Analysis of Optical Coherence Tomography Angiography
- Optical Coherence Tomography Angiography in Healthy Subjects
- Optical Coherence Tomography Angiography of Type 1 Neovascularization in Age-Related Macular Degeneration
- Optical Coherence Tomography Angiography of Type 2 Neovascularization in Age-Related Macular Degeneration
- Optical Coherence Tomography Angiography Features of Type 3 Neovascularization in Age-Related Macular Degeneration
- Optical Coherence Tomography Angiography of Mixed Neovascularizations in Age-Related Macular Degeneration
- Optical Coherence Tomography Angiography of Idiopathic Polypoidal Choroidal Vasculopathy
- Optical Coherence Tomography Angiography Study of Choroidal Neovascularization Early Response after Treatment
- Optical Coherence Tomography Angiography of Fibrosis in Age-Related Macular Degeneration
- Optical Coherence Tomography Angiography of Dry Age-Related Macular Degeneration
- Optical Coherence Tomography Angiography of Choroidal Neovascularization Secondary to Pathologic Myopia
- Optical Coherence Tomography Angiography of Diabetic Retinopathy
- New Findings in Diabetic Maculopathy and Proliferative Disease by Swept-Source Optical Coherence Tomography Angiography
- Optical Coherence Tomography Angiography of Retinal Artery Occlusion
- Optical Coherence Tomography Angiography of Retinal Vein Occlusion
- Optical Coherence Tomography Angiography of Deep Capillary Ischemia
- Optical Coherence Tomography Angiography of Macular Telangiectasia Type 2
- Optical Coherence Tomography Angiography in Dystrophies
- Swept-Source Optical Coherence Tomography Angiography of Paediatric Macular Diseases
- Optical Coherence Tomography Angiography of Miscellaneous Retinal Disease
- Subject Index