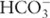![]()
1. Metabolic Synthesis
1.1 Background
In this chapter, we begin by providing a brief background on the classification of organisms. We then provide a general background on the synthesis of chemical biomarkers and their association with key metabolic pathways in organisms, as they relate to differences in cellular structure and function across the three systematic domains of life. We also discuss photosynthesis, the dominant pathway by which biomass is synthesized, and provide information about chemoautotrophic and microbial heterotrophic processes. This holistic view of biosynthetic pathways of chemical biomarkers provides a roadmap for other chapters in this book, where more specific details on chemical pathways are presented for each of the respective classes of biomarkers. While other excellent books in the area of organic geochemistry have effectively introduced the concepts of chemical biomarkers in the context of physical and chemical gradients found in natural ecosystems (e.g., anaerobic, aerobic) (Killops and Killops, 2005; Peters et al., 2005), we begin by first examining biosynthetic pathways at the cellular level of differentiation. We believe that an understanding of the general biosynthetic pathways of these chemical biomarkers is critical when examining the complexity of rate-controlling processes that determine their production and fate in aquatic systems. We also provide the major features of different cell membrane structures, since these membranes play an important role in the transport of simple molecules in and out of the cell and influence the preservation of chemical biomarkers in sediments.
1.2 Classification of Organisms
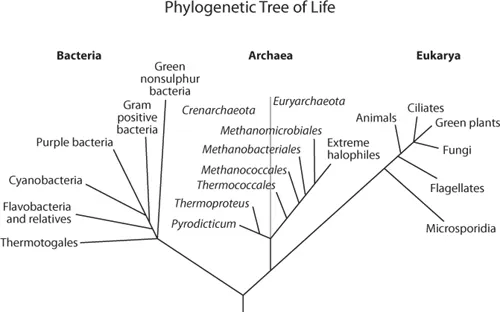
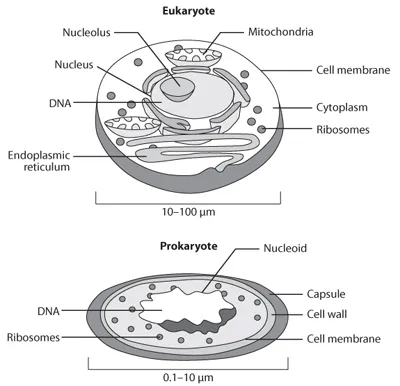
The taxonomic classification of all living organisms is shown in fig. 1.1. The former five-Kingdom classification system consisted of the following phyla: Animalia, Plantae, Fungi, Protista, and Bacteria. It was replaced by a three-domain system—primarily derived from the phylogenetic analysis of base sequences of nucleic acids from rRNA (Woese et al., 1990) (fig. 1.1). These domains can be further divided into heterotrophs (e.g., animals and fungi) and autotrophs (e.g., vascular plants and algae), which are either prokaryotes (unicellular organisms that do not possess a nuclear membranes [e.g., just a nucleoid, or DNA in the form of chromosomes] or eukaryotes (unicellular and multicellular organisms with nuclear membranes and DNA in the form of chromosomes) (fig. 1.2). The bacteria (eubacteria) and archaea (archaebacteria), both prokaryotes, represent important microbial groups and are involved in many of the biogeochemical cycling processes of aquatic ecosystems.
Figure 1.1. The three-domain system derived from the phylogenetic analysis of base sequences of nucleic acids from rRNA. Phylogenetically the Archaea fall into two distinct groups as shown by the gray line: the methanogens (and their relatives) and the thermophiles. (Adapted from Woese et al.,1990.)
Photosynthesis is the dominant process by which organic matter is synthesized. During photosynthesis, organisms synthesize simple carbohydrates (carbon fixation) using light energy, water and an electron donor. The earliest photosynthetic organisms are thought to have been anoxygenic, likely using hydrogen, sulfur, or organic compounds as electron donors. Fossils of these organisms date to 3.4 billion years (3.4 × 109 years or 3.4 Gyr) before present (BP). Oxygenic photosynthesis evolved later; the first oxygenic photosynthetic organisms were likely the cyanobacteria, which became important around 2.1 Gyr BP (Brocks et al., 1999). As oxygen accumulated in the atmosphere through the photosynthetic activity of cyanobacteria (Schopf and Packer, 1987), life on Earth needed to quickly adapt. In fact, it is believed that a transfer of bacterial genes was responsible for the development of the first eukaryotic cell (Gypania spiralis), estimated, based on the fossil record, to be 2.1 Gyr old (Han and Runnegar, 1992). When a cell consumed aerobic (oxygen-using) bacteria, it was able to survive in the newly oxygenated world. Today, the aerobic bacteria have evolved to include mitochondria, which help the cell convert food into energy. Hence, the appearance of mitochondria and chloroplasts are believed to have evolved in eukaryotes as endosymbionts (Thorington and Margulis, 1981). The current theory of endosymbionts is based on the concept that an earlier combination of prokaryotes, once thought to be living together as symbionts, resulted in the origin of eukaryotes (Schenk et al., 1997; Peters et al., 2005). This theory suggests that some of the organelles in eukaryotes (e.g., mitochondria, kinetosomes, hydrogenosomes, and plastids) may have begun as symbionts. For example, it is thought that mitochondria and chloroplasts may have evolved from aerobic nonphotosynthetic bacteria and photosynthetic cyanobacteria, respectively. Endosymbiotic theory also explains the presence of eubacterial genes within eukaryotic organisms (Palenik, 2002). The important energy-transforming processes that directly or indirectly occur in association with these organelles, such as photosynthesis, glycolysis, the Calvin cycle, and the citric acid cycle, are discussed in more detail later in this chapter.
Figure 1.2. The basic cellular design of Eukaryotes, unicellular and multicellular organisms with nuclear membranes and DNA in the form of chromosomes, and Prokaryotes, unicellular organisms that do not possess a nuclear membrane (e.g., just a nucleoid, or DNA in the form of chromosomes).
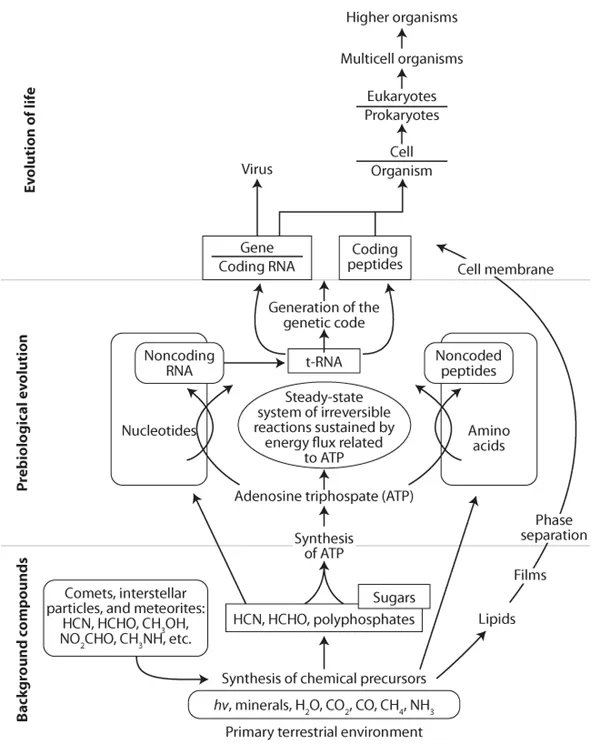
While the origins of life on early Earth remain controversial, experimental evidence for the possible evolution of early life began back with the work of Miller and Urey (Miller, 1953; Miller and Urey, 1959). The basic premise of this work, which has remained central in many current studies, is that simple organic compounds, including amino acids, formed after a spark was applied to a flask containing constituents thought to be present in the Earth’s early atmosphere (e.g., methane, ammonia, carbon dioxide, and water). It is thought that these simple organic compounds provided the “seeds” for the synthesis of more complex prebiotic organic compounds (
fig. 1.3). In addition to the in situ formation of these simple molecules on Earth (based on the aforementioned lab experiments in the 1950s), extraterrestrial sources, such as comets, meteorites, and interstellar particles, have also been posited as possible sources for seeding early Earth with these simple molecules. There is now strong evidence for the presence of these prebiotic compounds in these extraterrestrial sources (Engel and Macko, 1986; Galimov, 2006; and references therein). One particular theory focuses on the notion that the synthesis of adenosine triphosphate (ATP) was most critical in the early stages of prebiotic evolution (Galimov, 2001, 2004). The hydrolysis of ATP to adenosine diphosphate (ADP) is critical in the ordering and assembly of more complex molecules. For example, the formation of peptides from amino acids, and nucleic acids from nucleotides, is inherently linked to the ATP molecule (
fig. 1.3). Another important step in prebiotic chemical evolution of life was the development of a molecule that allowed for genetic coding in primitive organisms, and transfer ribonucleic acid (tRNA). Many scientists now suspect that all life diverged from a common ancestor relatively soon after life began (
figs. 1.1 and
1.3). Based on DNA sequencing, it is believed that millions of years after the evolution of archaebacteria and eubacteria, the ancestors of eukaryotes split off from the archaebacteria. The Earth is at least 4.6 Gyr old (Sogin, 2000), but it was the microbial organisms that dominated for the first 70 to 90 percent of Earth’s history (Woese, 1981; Woese et al., 1990). While there has been considerable debate about the composition of the atmosphere of early Earth, it is now generally accepted that it was a reducing environment (Sagan and Chyba, 1997; Galimov, 2005). Kump et al. (2010) state the atmosphere is now thought to have been composed primarily of N
2 and CO
2. Banded iron formations (BIF) first appear in sediments deposited 3 Gyr
BP, during the early history of the Earth. These formations include layers of iron oxides, either magnetite or hematite, alternating with iron-poor layers of shale and chert. It is thought that these iron oxide formations were formed in seawater from the reaction between oxygen produced during photosynthesis by cyanobacteria with dissolved reduced iron. The subsequent disappearance of banded iron formations in the geologic record approximately 1.8 Gyr
BP is believed to have resulted following a phase of rising oxygen levels in the atmosphere that began about 2.4 to 2.3 Gyr
BP (Farquhar et al., 2000; Bekker et al., 2004). Other recent work has suggested that even during the mid-Proterozoic (ca. 1.8 to 0.8 Gyr ago) the oceans were either
anaerobic (no oxygen) or
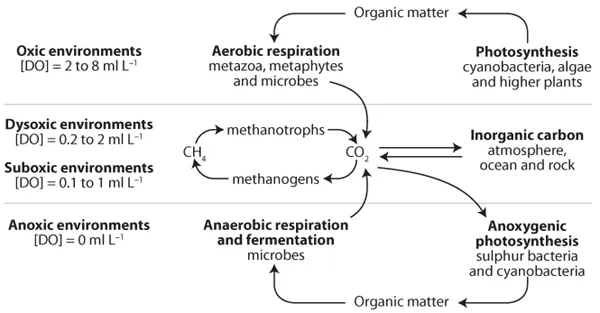
dysaerobic (low oxygen) compared to the oceans of today (Arnold et al., 2004) (
fig. 1.4). The increase in oxygen in the oceans from this time period to the present was due to increased production by photoautotrophic organisms, which transfer atmospheric CO2 and/or dissolved inorganic C (e.g.,
) into biomass using photosynthesis; many of these early photoautotrophs were likely cyanobacteria (Brocks et al., 1999).
Figure 1.3. A scenario for the evolution of life focused on the notion that the synthesis of adenosine triphosphate (ATP) was most critical in the early stages of prebiotic evolution. (Adapted from Galimov, 2001, 2004.)
Figure 1.4. Schematic depicting the redox conditions during the mid-Proterozoic (ca. 1.8 to 0.8 Gyr ago) in the oceans, which are believed to have been considerably more anaerobic (no oxygen) and dysaerobic (low oxygen) than the oceans of today. (Adapted from Arnold et al., 2004.)
Archaebacteria were originally thought to live only in extreme environments (e.g., high temperatures, pH extremes, and high radiation levels), but have recently been found in a variety of habitats (Delong and Pace, 2001; Giovannoni and Stingl, 2005; Delong, 2006; Ingalls et al., 2006). Examples of archaebacteria include anaerobic methanogens and halophilic bacteria, and consist of organisms living both in cold (e.g., Antarctica) and hot (e.g., springs of Yellowstone Park [USA]) environments. The fact that these organisms can be found in extreme environments is consistent with the ancient heritage of this domain. Early Earth was likely a very hot environment, with many active volcanoes and an atmosphere composed mostly of nitrogen, methane, ammonia, carbon dioxide, and water—with little to no oxygen present. It is believed that the archaebacteria, and in some cases bacteria, evolved under these conditions, allowing them to live in harsh conditions today. For example, thermophiles live at high temperatures, of which the present record is 121°C (Kashefi and Lovley, 2003). In contrast, no known eukaryote can survive over 60°C. Another example is the psychrophiles, which live in extremely cold temperatures—there is one species in the Antarctic that grows best at 4°C. As a group, these hard-living archaebacteria are called extremophiles. The archaebacteria include other kinds of extremophiles, such as acidophiles, which live at pH levels as low as 1. Alkaliphiles thrive at high pH levels, while halophiles live in very salty environments. It should be noted that there are also alkaliphilic, acidophilic, and halophilic eukaryotes, and that not all archaebacteria are extremophiles. It has also been suggested that the thermophilic archaebacteria that live around deep-sea volcanic vents may represent the earliest life on Earth (Reysenbach et al., 2000). These thermophilic archaea harvest their energy very efficiently from chemicals (e.g., H2,CO2, and O2) found at the vents using a process called chemosynthesis. These organisms are not grea...