![]()
1
PHASE CHANGES
1.1 Complexity
By tracking the history of life and society, we find much evidence of deep changes in life forms, ecosystems, and civilizations. Human history is marked by crucial events such as the discovery of the New World in 1492, which marked a large-scale transformation of earth’s ecology, economics, and culture (Fernandez-Armesto 2009). Within the context of biological change, externally driven events such as asteroid impacts have also triggered ecosystem-scale changes that deeply modified the course of evolution. One might easily conclude from these examples that deep qualitative changes are always associated with unexpected, rare events. However, such intuition might be wrong. Take for example what happened around six thousand years ago in the north of Africa, where the largest desert on our planet is now located: the Sahara. At that time, this area was wet, covered in vegetation and rivers, and large mammals inhabited the region. Human settlements emerged and developed. There are multiple remains of that so-called Green Sahara, including fossil bones and river beds. The process of desertification was initially slow, when retreating rains changed the local climate. However, although such changes were gradual, at some point the ecosystem collapsed quickly. The green Sahara became a desert.
Transitions between alternate states have been described in the context of ecology (Scheffer 2009) and also in other types of systems, including social ones. Complex systems all display these types of phenomena (at least as potential scenarios). But transitions can also affect, sometimes dramatically, molecular patterns of gene activity within cells, behavioral patterns of collective exploration in ants, and the success or failure of cancer or epidemics to propagate (Solé et al. 1996; Solé and Goodwin 2001). When a given parameter is tuned and crosses a threshold, we observe change in a system’s organization or dynamics. We will refer to these different patterns of organization as phases. The study of complexity is, to a large extent, a search for the principles pervading self-organized, emergent phenomena and defining its potential phases (Anderson 1972; Haken 1977; Nicolis and Prigogine 1977, 1989; Casti 1992a, b; Kauffman 1993; Cowan et al. 1994; Gell-Mann 1994; Coveney and Highfield 1995; Holland 1998; Solé and Goodwin 2001; Vicsek 2001; Mikhailov and Calenbuhr 2002; Morowitz 2002; Sornette 2004; Mitchell 2009). Such transition phenomena are collective by nature and result from interactions taking place among many interacting units. These can be proteins, neurons, species, or computers (to name just a few).
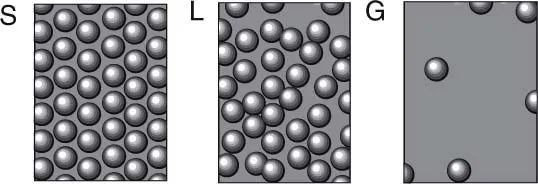
In physics, phase changes are often tied to changes between order and disorder as temperature is tuned (Stanley 1975; Binney et al. 1992; Chaikin and Lubensky 2001). Such phase transitions typically imply the existence of a change in the internal symmetry of the components and are defined among the three basic types of phases shown in figure 1.1. An example of such transition takes place between a fluid state, either liquid or gas, and a crystalline solid. The first phase deals with randomly arranged atoms, and all points inside the liquid or the gas display the same properties. In a regular (crystalline) solid, atoms are placed in the nodes of a regular lattice. In the gas phase (at high temperature) kinetic energy dominates the movement of particles and the resulting state is homogeneous and isotropic. All points are equivalent, the density is uniform, and there are essentially no correlations among molecules. In the liquid phase, although still homogeneous, short-distance interactions between molecules leads to short-range correlations and a higher density. Density is actually the fundamental difference distinguishing these two phases. Finally, the ordered arrangement observed at the solid phase is clearly different in terms of pure geometry. Molecules are now distributed in a highly regular way. Crystals are much less homogeneous than a liquid and thus exhibit less symmetry.
Figure 1.1. Three standard phases of matter: solid (S), liquid (L) and gas (G). Increasing a given external parameter (the control parameter) such as temperature, we can increase the degree of disorder. Such disorder makes molecules fluctuate around their equilibrium positions, move around them, or just wander freely.
However, beyond the standard examples of thermodynamic transitions between these three phases, there is a whole universe. Matter, in particular, can be organized in multiple fashions, and this is specially true when dealing with so-called soft matter. But the existence of different qualitative forms of macroscopic organization can be observed in very different contexts. Many are far removed from the standard examples of physics and chemistry. And yet, some commonalities arise.
1.2 Phase Diagrams
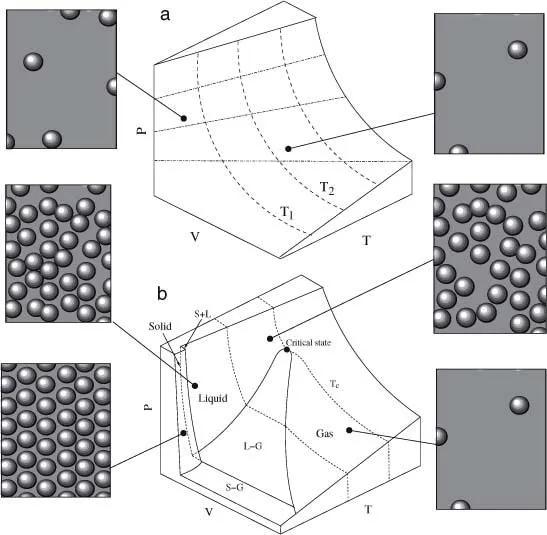
Phase changes are well known in physics: boiling or freezing are just two examples of changes of phase where the nature of the basic components is not changed. The standard approach of thermodynamics explores these changes by defining (when possible) the so-called equation of state (Fermi 1953), which is a mathematical expression describing the existing relations among a set of state variables (i.e., variables defining the state of a system). For an ideal gas, when no interactions among molecules need be taken into account,1 the equation reads
where p, V, and T indicate pressure, volume, and temperature, respectively. Here R is the so-called gas constant (the same for all gases) and n the amount of substance (in number of moles). This equation is valid for a pure substance, and as we can see, it establishes a well-defined mathematical relation between p, V, T, and n. Given this equation, only three independent variables are at work (since the fourth is directly determined through the state equation). From this expression, we can plot, for example, pressure as a function of V and T:
which describes a continuous surface, displayed in figure 1.2a. For a given amount of perfect gas n, each point on this surface defines the only possible states that can be observed. Using this picture, we can consider several special situations where a given variable, such as temperature, is fixed while the other two can be changed. Fixing a given temperature T1, we can see, for example, that pressure decays with volume following an inverse law, that is, p(V; T1) = nRT1/V , which allows defining an isothermal process as the one moving on this line. The curve is called an isotherm. By using different temperatures, we can generate different isotherms (which are in fact cross sections of the previous surface). Similarly, we can fix the volume and define another set of curves, now given by p(T) = BT with B = nR/V . The important idea here is that all possible states are defined by the equation of state and that in this case all possible changes are continuous.
Figure 1.2. The equation of state describes the relations between the three key variables (p, V, T ) that allow definition of a surface. In (a) the corresponding surface p(V, T ) for an ideal gas is shown. This is obtained from the equation of state pV = nRT, which gives a continuous surface associated to a single gas phase. In reality (b) things are much more complex and the surface is discontinuous. This second plot corresponds to a substance (such as water) that expands when it solidifies. At high temperatures it is steam. Here kinetic energy is larger than the potential energy. But once T is decreased, nonlinear changes occur, as is evident from the discontinuous shape of the surface.
The previous equations are valid when we consider a very diluted gas at high temperature. However, in the real world, transitions between different macroscopic patterns of organization can emerge out of molecular interactions2 (figure 1.2b). Different phases are associated with different types of internal order and for example when temperature is lowered, systems become more ordered. Such ...