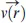1.1 THE COFFEE-STAIN EFFECT
Drinking a cup of coffee, especially a good Italian espresso, is one of those little pleasures of everyday life. It can be difficult to imagine, therefore, a small droplet of our coffee as the starting point of a journey into self-assembly and nanotechnology as we try to enjoy our moments of relaxation. However, there is so much interesting and unexpected physics and chemistry behind the evaporation of a coffee droplet that it is worth observing the details of this phenomenon. The coffee-stain effect is intriguing because after the evaporation, what is left behind is not a homogeneous halo. It is a ring (Figure 1.1). This is a general effect as it does not matter what kind of solution is used as the coffee stain seems to be an ubiquitous phenomenon. The effect can be reproduced using different solvent−solute combinations, which comprise single molecules1, nanoparticles2, polymers3, salts4 and even bacteria; it is also observed at different length scales, from micron-sized particles to nano-objects as well as on the molecular level. It can also be observed on different types of substrates, such as silicon, glass, metal, mica and concrete. The coffee stain effect may represent an issue if homogeneous spots are required. For instance, without preventing such an effect, the ink-jet technology would produce dishomogeneous plots5,6. A detailed understanding of the formation mechanism is necessary either to prevent the formation of the coffee-stain effect, or to take advantage of it in the development of new applications.
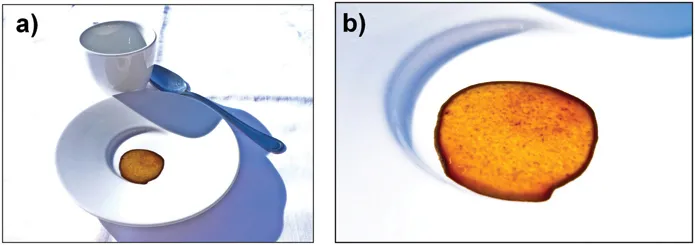
Figure 1.1 Dropping a coffee droplet on a solid substrate leaves upon evaporation a typical stain with a ring b) Enlarged image of the coffee stain presenting the typical ring shape (pictures by Laura Villanova).
The first comprehensive explanation was reported by Deegan and co-workers in an article published in Nature (1997) and it is based on the observation that a mechanical constraint of an evaporating droplet causes a capillary flow towards the contact line7. When we pour a liquid droplet and observe how its evaporation occurs two different scenarios can be envisaged: in the first case the droplet is free to move on the substrate and in the second case the surface irregularities cause a blockage of the contact line that remains pinned until the evaporation process has gone almost to completion.
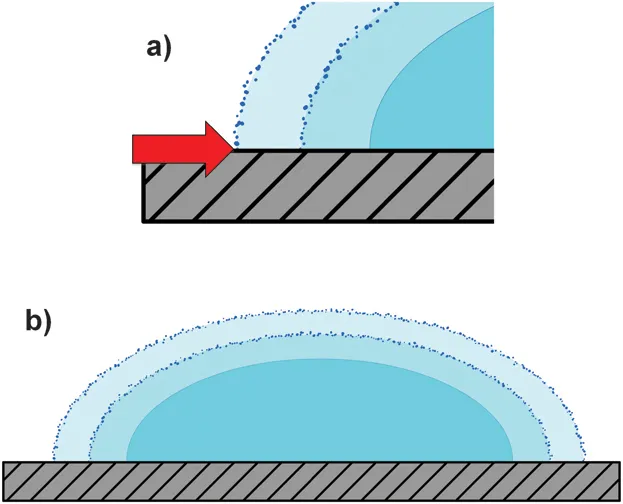
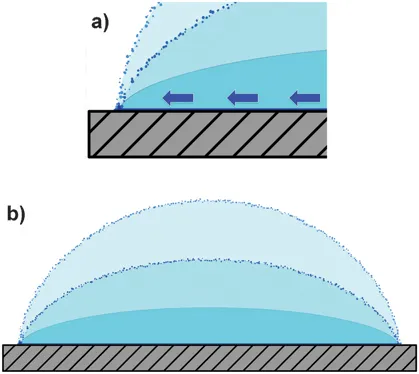
In the first case, therefore, the contact line is not pinned and the droplet shrinks; the profile of the droplet will not change while its radius, R, decreases (Figure 1.2). In contrast if the contact line is pinned8 the droplet will flatten modifying its profile while the liquid evaporating from the edge is replenished by the liquid from the interior (Figure 1.3). If the liquid contains dispersed particles they will be carried out to the edge of the droplet by capillary flow that is induced by the pinning. The mass accumulation at the contact line then forms a ring-like structure around the droplet.
Figure 1.2 Evaporation without flow. (a) The contact line is not pinned and moves in the direction indicated by the red arrow; the droplet shrinks. (b) Evaporation occurs over the entire drop surface and if the contact line is free to recede, the drop profile is preserved during evaporation.
Figure 1.3 Evaporation without flow. (a) The contact line is pinned and the droplet does not shrink. A compensating flow (in the direction of the blue arrows) is needed to keep the contact line fixed; (b) the drop profile is not preserved during evaporation.
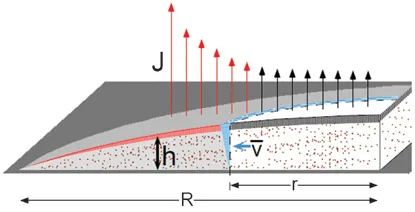
We can have a closer look at the evaporation process as described by Deegan
9 for an ideal case of a small, thin, dilute and circular drop with a fixed radius,
R. The contact line is pinned and at every point of the droplet,
r, and there is an evaporative flux,
J(
r), which reduces the height,
h(
r), of the droplet. The evaporation rate, however, is not
uniform and is greater when close to the edge of the contact line. If the contact line is not pinned, what is observed is a decrease of the radius while the overall profile of the droplet, as we have just seen, remains constant with time. However, if the contact line is pinned, the high evaporation at the edge should be somehow compensated by an outward flow. During this process the profile of the droplet should maintain its spherical shape, which is governed by the surface tension of the liquid. In a unit of time, Δ
t, the volume, which is removed, produces a decrease in the droplet height,
h(
r), which is higher toward the center. If, however, we look to the flux it is larger at the edge (red arrows in the
Figure 1.4) and in this case it would be expected that the volume of liquid that is removed close to the contact line during evaporation is greater and not smaller with respect to the center of the droplet. This observation can be explained if we assume a flow height of average speed
exists and is directed towards the droplet edge. This flow height keeps the contact line fixed and balances the differences in the evaporation rate at different points within the droplet.
Figure 1.4 The outward evaporation flow in a circular pinned droplet of radius,
R. The evaporative flow,
J, indicated in the figure by the arrows, produces a shrinkage of the droplet which changes the profile and reduces its height,
h(
r) at every point
r. The volume of the striped region corresponds to the volume removed by
J with evaporation; in the shaded region, however, the red-striped volume is smaller than the volume removed by the local flux
J (red arrows), which is higher with respect to the center of the droplet. The deficit volume has to be compensated by a liquid flow: in a time Δ
t the fluid at
r sweeps out the blue-striped region; its volume is the deficit volume and
is its depth-averaged speed. (Reprinted by permission from Macmillan Publishers Ltd:
Nature, 1997,
389, 827, copyright 1997.)
Why is the evaporative flow at the edge of the contact line enhanced? This is a complex problem which is similar to the case of an electric field near the sharp edge of a conductor. When close to the contact line, the evaporative flow diverges and is dependent on the relative distance from the center of the droplet and the contact angle7,9:
J (r, t) ∼ (R − r)−λ (1.1)
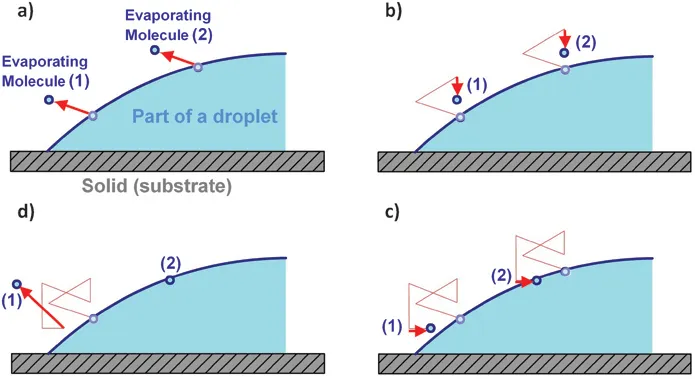
where λ = (π − 2θc)/(2π − 2θc) and θc is the contact angle of the liquid with the solid substrate. The reason for the higher evaporation rate at the edge is because of the greater probability of escaping, which a molecule that is close to the contact line has than a molecule at the center of the droplet. As we can see in Figure 1.5, the curvature at the edge decreases the probability of an evaporating molecule to be reabsorbed after evaporation. The same random path of an evaporating molecule at the center or at the edge of the droplet gives two different results: reabsorbing or escaping.
Figure 1.5 The probability of an evaporating molecule to be reabsorbed after evaporation depends on its position at the liquid−air interface. The same random path of an evaporating molecule at the edge (molecule 1) or at the center (molecule 2) of the droplet (a and b) gives two different results, reabsorbing or escaping (c and d).
It should be noted that while pinning the droplet on the substrate at the beginning is activated by surface roughness and irregularities, a ‘self-pinning’ effect is also intrinsic to ring formation8. The accumulation of matter at the droplet edge increases the energy barrier that the contact line has to overcome in order to move.
After the work of Deegan, the physics of the coffee-stain phenomenon has been widely investigated and several complex models, which consider the influence of different parameters have been described. Further reading on the subject can be found in references10–15.