1.1 Nickel Utilization
1.1.1 Nickel in Biology
Nickel was first identified in the 1960s as an essential nutrient for strains of Hydrogenomonas that were growing chemolithotrophically on the simple gasses H2, O2, and CO2.1 This discovery was followed by the observation that Methanobacterium thermoautotrophicum also required nickel supplementation of the growth media, but only if the media had not had any contact with stainless steel equipment, suggesting that even the trace nickel leaching from transient contact with syringe needles was sufficient for healthy growth.2 A clear function for nickel was established with the finding that nickel is an essential component of Jack Bean urease,3 which explained the nickel dependence of soybeans grown with urea as the nitrogen source.4 Subsequently, it was found that nickel is required for the production of carbon monoxide dehydrogenase in Clostridium pasteurianum.5 It is now well established that nickel is an essential cofactor for a variety of enzymes, many of which have been described in earlier reviews (e.g., see ref. 6–10).
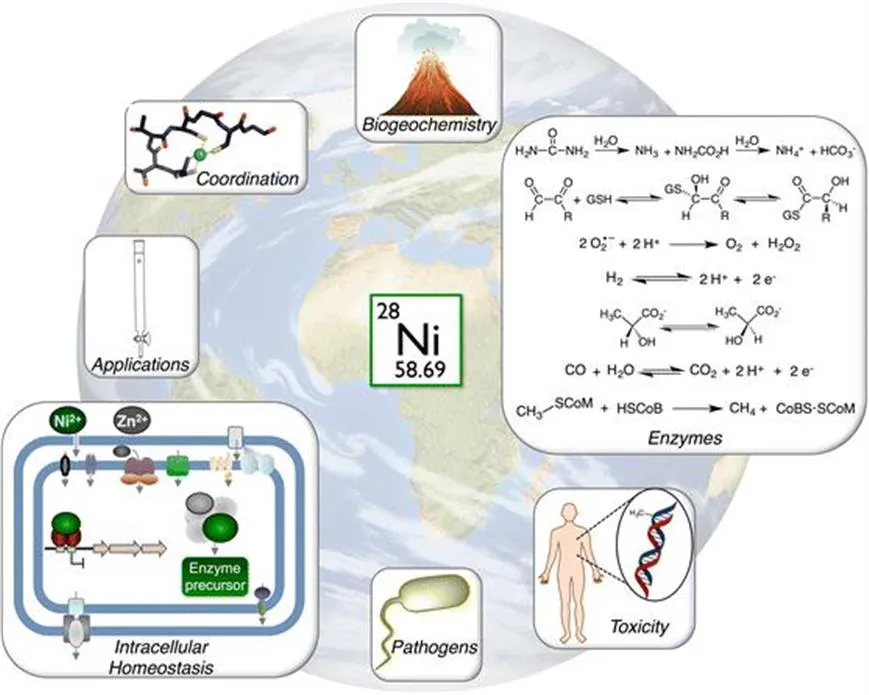
On a global scale, the importance of nickel in biology is highlighted (Figure 1.1) by the discussion in Chapter 2 about the influence of nickel-utilizing organisms on the earth’s geochemistry, and vice versa. It is striking that many nickel enzymes consume or produce small molecule gasses, they are often required for anaerobic microbial metabolism, and they contribute to the global elemental cycles.11–13 These types of chemistry highlight the ancient history of nickel as a biological cofactor for organisms that evolved early on, during the origins of life as we know it.14 In fact, given that nickel-enzyme metallocenters are often complex, multi-metal clusters, it has been suggested that these metal complexes may mimic the minerals that catalyzed the first abiotic reactions where life emerged.15,16 The link between the evolution of nickel use and nickel availability is exemplified by the presence of nickel superoxide dismutase (NiSOD) in many marine organisms (Chapters 2 and 9). SODs provide critical protection from reactive oxygen molecules that are byproducts of living in oxygen-containing environments, and there are three distinct versions of this enzyme that catalyze the same reaction but that contain different types of metal.17 It is likely that NiSOD was favored in some types of settings, such as marine environments, over other metal-utilizing SODs because nickel was more accessible than other types of metals, but that development may in turn have resulted in nickel depletion of those same environments (Chapter 2).
Figure 1.1 The global influence of the biological chemistry of nickel. Nickel plays a central role in biology as a cofactor in a wide variety of enzymes. The evolution of nickel as a nutrient in living organisms is intimately linked to the earth’s geochemistry, and the intracellular use of nickel is supported by many additional nickel proteins that maintain the availability and distribution of the metal ions. The study of these nickel systems has revealed extensive information about the coordination chemistry of nickel proteins, and applications of this knowledge include biotechnology tools and potential targets for new antimicrobials. However, nickel exposure is also toxic, which can be a problem in all organisms including those for which a nutritional requirement for nickel has not been identified, such as humans.
1.1.2 Nickel in Humans
As of yet, a required bioactivity for nickel has not been identified in human cells. However, nickel is ingested in many types of food,18,19 and nickel deprivation has a negative impact on various physiological factors in animals.20,21 Nickel circulates bound to serum albumin, as well as in trace amounts to several types of amino acids and α2-macroglobulin (nickeloplasmin),22 and is widely distributed in tissues throughout the body.21
Human exposure to nickel is also impacted by anthropogenic activities, such as metal-related industries and industrial pollution.19 This exposure can lead to toxicity, an occupational hazard of industries such as nickel mining and refining or stainless steel manufacturing and manipulation, with nickel being a well-established carcinogen.19 Chapter 3 provides an extensive discussion about the significant epigenetic consequences of nickel exposure in humans, such as changes to DNA methylation patterns and to levels of microRNAs, both of which promote carcinogenesis. Nickel is also an allergen, and everyday exposure to nickel in common household items, such as cooking utensils, jewelry, and money, can cause allergic reactions.23 For example, in the early 2000s the Euro coins elicited strong skin reactions in people with nickel allergies, possibly due to solubilization of the metal by human sweat.24,25 Later on, legislation was introduced to limit the amount of nickel contained in manufactured items in an effort to reduce human skin contact with nickel.26 The mechanisms of allergic reactions to nickel are multifaceted,25,26 but activation of the human Toll-like receptor 4 has a key role in the inflammatory response to nickel.27 Furthermore, modeling and mutagenesis of several non-conserved histidines revealed the location of a putative nickel ion binding site on TL4, and resolved the long standing mystery behind different nickel sensitization in humans versus in mice. These results also raise the questions of whether there is some type of selection pressure for the distinct sequence motif in human TL4, and why all humans are not sensitized to nickel.
1.2 Nickel Enzymes
Multiple nickel enzymes are found in Nature. Consideration of how nickel ions interact with the enzyme proteins, as well as the proteins that support nickel homeostasis, has revealed some common themes about these complexes. Chapter 4 surveys our knowledge about the coordination chemistry of nickel in biology, which has been greatly augmented by the study of smaller peptide nickel complexes. Some binding preferences can be extracted, and these preferences are emphasized in proteins dedicated to nickel storage and distribution. However, it is clear that nickel is not inordinately exclusive and can be comfortably accommodated by a variety of protein sites. Furthermore, as shown below, nickel catalyzes a wide range of chemical reactions when bound to enzyme proteins. Our current understanding about many of these nickel enzymes is discussed in detail in Chapters 5–11 of this book, along with outstanding questions.
As mentioned above, the first enzyme that was recognized to use nickel as a cofactor was urease, which catalyzes the hydrolysis of urea.3 This enzyme is a key virulence factor in multiple pathogenic bacteria, as highlighted in Chapter 16. The products of urease provide a source of nitrogen, buffer the local microenvironment, and/or contribute to pathogenesis via the formation of infection stones. Furthermore, as discussed in Chapter 5, urease can also be a significant agricultural problem. Chapter 5 describes the many structural and enzymatic studies performed with urease and various inhibitors, which together form the foundation for a detailed mechanism of action. This knowledge can now be used to guide the design of better inhibitors, and also informs the discussion of why nickel is used in urease, as opposed to more commonly available metal ions that, at first glance, appear suitable for catalysis of this chemical reaction.
Another nickel enzyme is [NiFe]-hydrogenase, which catalyzes the relatively simple reversible reaction between two protons and two electrons to generate hydrogen gas; the direction of the reaction depends on the biochemical context of individual enzymes.28,29 However, Chapter 6, which focuses on the results from X-ray crystallography, describes a complex system that involves multiple enzyme states. Furthermore, several mechanisms by which [NiFe(Se)]-hydrogenase enzymes prevent or deal with inactivation by poisons such as oxygen have been uncovered. This enzyme is also highlighted in Chapter 16 as a virulence factor for several human pathogens.
Chapter 7 discusses the extensive structural and biochemical studies that are unveiling what happens at the metal clusters of carbon monoxide dehydrogenase and acetyl coenzyme A synthase.30 These enzymes catalyze one-carbon chemistry that is a part of the basic metabolism of many organisms, serving as the entry or exit point of CO2 and/or CO. This chapter also highlights the critical functions provided by the extensive protein scaffolds, which facilitate the handling of the gaseous and potentially toxic small molecules involved. The ramifications of the possible applications of this chemistry, either through harnessing the enzymology or mimicking Nature’s strategies, in terms of generating solutions to global climate change and sources of sustainable energy are considerable.
The central role of nickel in the global carbon cycle is also emphasized in Chapter 8, which describes methyl coenzyme ...