
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Maths for Chemists
About this book
The two volumes of Maths for Chemists provide an excellent resource for all undergraduate chemistry students but are particularly focussed on the needs of students who may not have studied mathematics beyond GCSE level (or equivalent). The texts are introductory in nature and adopt a sympathetic approach for students who need support and understanding in working with the diverse mathematical tools required in a typical chemistry degree course. The early chapters of Maths for Chemists Volume I: Numbers, Functions and Calculus provide a succinct introduction to the important mathematical skills of algebraic manipulation, trigonometry, numbers, functions, units and the general grammar of maths. Later chapters build on these basic mathematical principles as a foundation for the development of differential and integral calculus. In spite of the introductory nature of this volume, some of the more important mathematical tools required in quantum chemistry are deliberately included, through a gradual introduction to, and development of, the concept of the eigenvalue problem. Ideal for the needs of undergraduate chemistry students, Tutorial Chemistry Texts is a major series consisting of short, single topic or modular texts concentrating on the fundamental areas of chemistry taught in undergraduate science courses. Each book provides a concise account of the basic principles underlying a given subject, embodying an independent-learning philosophy and including worked examples.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
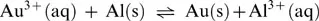
1.1 Real Numbers
1.1.1 Integers
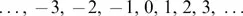
Table of contents
- Cover image
- Title page
- Copyright
- Preface
- Contents
- 1 Numbers and Algebra
- 2 Functions and Equations: Their Form and Use
- 3 Limits
- 4 Differentiation
- 5 Differentials
- 6 Integration
- 7 Differential Equations
- 8 Power Series
- 10 Working with Arrays I: Determinants
- 11 Working with Arrays II: Matrices and Matrix Algebra
- 12 Vectors
- 13 Simple Statistics and Error Analysis
- Worked Answers to Problems
- Glossary
- Subject Index
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app