1.1 Introduction
Biocatalysis, by which we mean the use of enzymes and microorganisms as catalysts for chemical transformations, has had a long and distinguished history, particularly in the brewing, baking and animal feed industries. In addition, the development of fermentation-based processes for antibiotic production relies squarely on the fundamental properties of enzymes for the production of natural products. In the latter half of the 20th century, biocatalysis started to be viewed as a potential route to unnatural or synthetic compounds. For example, scientists in Schering in Berlin in Germany discovered that semi-synthetic steroids could be manipulated with exquisite selectivity using enzymes. In the 1970s, the use of hydrolytic enzymes (e.g. lipases and proteases) and oxidoreductases (e.g. alcohol dehydrogenases) started to gain popularity for the synthesis of chiral building blocks, particularly within the pharmaceutical industry where stereochemical purity in the final product was a high priority. The discovery that some enzymes (e.g. lipases and proteases) could be used under low water conditions in organic solvents extended the range of these biocatalysts to non-polar organic substrates. However, by the early 1990s, the range of biocatalysts being employed by organic chemists was still relatively narrow and confined to hydrolases and oxidoreductases. A typical international conference on biocatalysis at that time would focus ca. 80–90% of the presentations on these two classes of enzymes.
In the 1990s, several major developments occurred within the field of biocatalysis that together changed the face of the discipline and resulted in a rapid diversification of the enzymes available to synthetic chemists.1 Firstly the use of molecular biology protocols as tools to clone, express and manipulate novel genes, and hence produce new enzymes, became much more widespread and meant that there was increasingly less reliance on the use of wild-type crude cell extracts for biocatalysis. Secondly, the use of protein engineering and directed evolution emerged as powerful strategies for optimisation of the properties of enzymes, particularly in the context of improving stability, stereoselectivity, substrate tolerance and catalytic activity. Thirdly, the availability of sequenced genomes meant that there was an explosion in the number of gene/protein sequences that could be mined and used as the basis for discovering new enzymes. At the beginning of 2000, the cost of DNA sequencing and gene synthesis began to drop considerably, as a result of technological advances, which meant that ordering synthetic genes became cost effective and a rapid way of generating novel biocatalysts for screening. Together, these new technologies resulted in a significantly broader range of enzymes becoming available to organic chemists. Nowadays, we have a plethora of different methods and approaches available to us for the discovery and development of new biocatalysts (Figure 1.1). The challenge has shifted from simply discovering new enzymes to trying to curate the existing databases in order to try to understand which sequences might code for which enzyme activity. De novo protein design has made major strides forward, and allows us to generate new enzyme activities from alternative scaffolds. Enzyme evolution has developed to the point where it is now routinely applied to optimise enzyme performance and represents one of the most powerful algorithms available to those interested in the development of new biocatalysts. Other scientific advances in the scale-up of biocatalytic processes provided greater levels of confidence that once a biocatalyst had been identified for a specific chemical transformation, there was a good chance of developing a practical large-scale process. Biocatalysis is now a maturing technology for the manufacture of a range of chemical products across a wide range of industries from pharmaceuticals and biofuels to polymers and personal healthcare products. Compared to traditional synthesis methodologies, biocatalysis offers a number of advantages that can contribute to more sustainable manufacturing processes. In addition, the emerging field of synthetic biology offers the potential for increasingly efficient synthesis by combining multiple biocatalytic reactions all within a single host organism.
Figure 1.1 Modern technologies available for enzyme discovery, development and application in organic synthesis.
Today, biocatalysts are increasingly being considered as an option when planning synthetic strategies for the construction of target organic molecules, particularly in cases where it is important to try to achieve some type of selectivity (e.g. stereo-, regio-, and chemoselectivity). The use of biocatalysts as an alternative to chemical reagents and catalysts can also provide benefits in terms of a reduced number of synthetic steps, lower costs of goods, reduced use of harmful solvents and improved safety profiles. Together, these benefits can lead to a more sustainable overall process with a lower overall environmental impact. However, a major barrier remains preventing the more widespread application of biocatalysis, namely a general lack of awareness and understanding amongst the synthetic organic community regarding the types of biocatalysts that are available and the various ways in which they can be applied in target molecule synthesis.
During the past ten years, a number of excellent books and reviews have been published highlighting the various biocatalysts that are now available and detailing the types of transformations that can be accomplished.2 Typically, these books are organised according to different classes of enzyme (e.g. hydrolases, dehydrogenases, transferases and lyases) and the various reactions are presented based upon the type of synthetic transformation that can be achieved (e.g. hydrolysis, reverse hydrolysis, C–C bond formation, C–X bond formation, oxidation, etc.). This approach provides the reader with an excellent overview of where specific biocatalysts can now be applied in organic synthesis, together with an insight into the tolerance of enzymes for unnatural substrates, which allows consequently for the synthesis of non-natural target molecules. However, as more new biocatalysts become available for application in synthesis, inevitably the question arises as to what is the best way of preparing a particular target molecule, or a building block with specific arrangements of functionality and chirality, given the fact that there may be several available options. This situation is particularly true for the synthesis of chiral alcohols, amines, carboxylic acids, esters, amides, etc., for which there are now several available biocatalytic systems that could in principle be used, and certainly many more than were available ten years ago. As more distinct biocatalytic platforms are developed, this expansion of options will continue and indeed accelerate. Indeed, our ability to discover new biocatalysts, and subsequently engineer them for broader substrate tolerance or new reaction chemistry, is rapidly increasing due to advances in technologies such metagenomics, genome sequencing, protein engineering, directed evolution and high-throughput screening.
Despite the now widespread application of retrosynthesis in everyday organic synthesis, there has been no systematic attempt to include biocatalytic reactions in the whole process of synthetic planning and design. This omission is more remarkable in view of the increasing interest in using biocatalysts for synthesis in both industry and academia. Students are typically taught retrosynthetic analysis at around the same time as more traditional C–C bond forming methods. As a result, the connection between biocatalysis and synthesis tends to be made towards the end of their studies, generating the perception that enzymes are specialised and not suitable for mainstream organic synthesis. If biocatalysis is to be fully incorporated into the synthetic chemist's toolbox, then the teaching of biocatalytic methodology and retrosynthesis must be concurrent, as it is with more traditional organic synthesis.
1.2 Biocatalytic Retrosynthesis
In the mid-1960s, synthetic organic chemists began to apply the principles of retrosynthetic analysis during the planning of new routes to target molecules. Retrosynthetic analysis, which was originally proposed by E. J. Corey, fundamentally changed the way in which synthetic chemists conceived and developed approaches to the total synthesis of natural and unnatural products.3 An important principle of retrosynthetic design is that each disconnection in the reverse sense must represent a feasible transformation in the forward synthesis direction. Students are generally taught to disconnect molecules by a formalised process of bond cleavage of either C–C or C–X bonds in the reverse direction. In addition, functional group interconversions (FGI's) are used to prepare the molecule for the desired bond cleavage. An important by-product of this method of analysis has been that synthetic chemists have discovered disconnections for which the corresponding forward synthesis methodologies were unknown and new additions to the synthesis toolbox have been developed as a result.
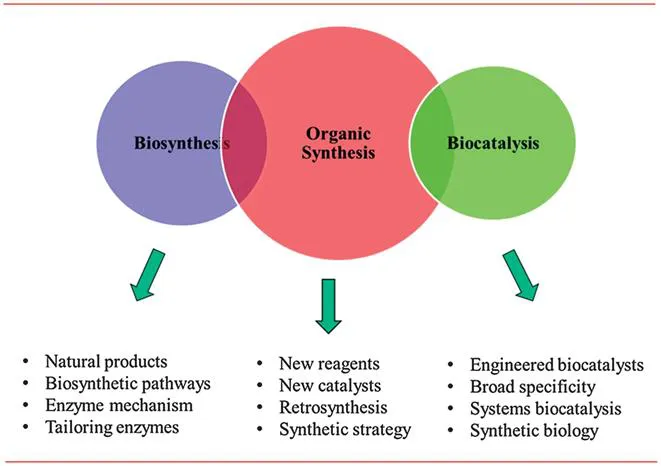
The invention of new reagents and increasingly catalysts for selective organic synthesis has resulted in the situation where today there is (almost) no molecule that is beyond the reach of the synthetic organic chemist. Organic synthesis using synthetic reagents and catalysts is the predominant technology for the manufacture of a broad range of chemical products, ranging from pharmaceuticals and agrochemicals to polymers and plastics, and including a diverse range of specialty chemicals in between (Figure 1.2). Organic synthesis by and large uses feedstocks derived from the petrochemical industry and indeed to a large extent the two disciplines have ‘co-evolved’ – one provides the starting materials for the other – and new chemical reagents and catalysts are designed to be compatible with oil-derived starting materials.
Figure 1.2 The ‘three’ approaches to organic synthesis.
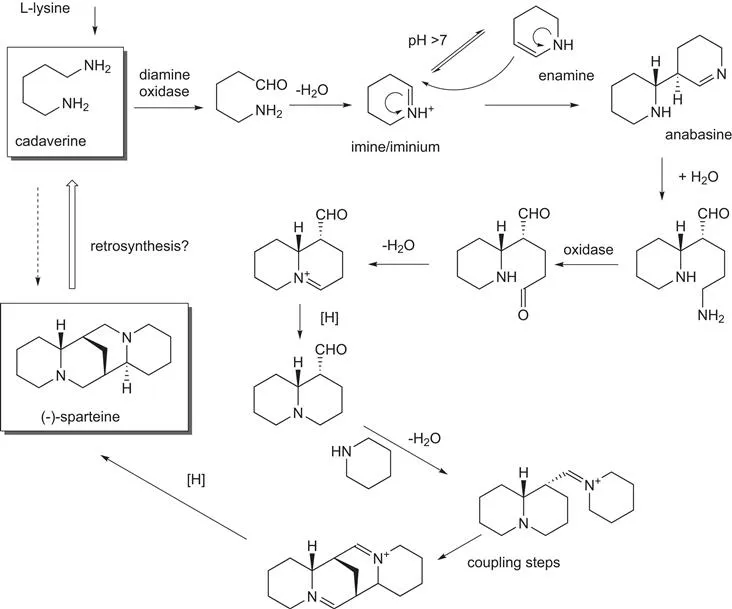
Figure 1.2 presents two alternative scenarios for undertaking organic synthesis. As noted earlier, many semi-synthetic pharmaceuticals are derived from natural products derived from fermentation processes. Over millions of years, Nature has evolved complex biosynthetic pathways for the production of a truly diverse set of natural products, including alkaloids, polyketides, terpenes, carbohydrates, amino acids, etc. Even some of the simpler natural products, such as the alkaloid (−)-sparteine, are fantastic examples of the way in which biosynthetic pathways are able to convert simple building blocks, in this case cadaverine, to structurally more complex target molecules using simple chemical transformations (Figure 1.3). Inspection of this pathway reveals a typical scenario in the biosynthesis of natural products, i.e. the enzymes involved are catalysing simple chemical transformations, e.g. oxidation, reduction, and hydrolysis. The bond forming and bond breaking processes involved are not sophisticated, but it is the way in which the reactions are orchestrated that results in the rapid construction of molecular complexity from simple achiral precursors. And of course, all of the reactions are subjected to catalysis by an enzyme – that is Nature's way. Imagine being asked to make a molecule such as (−)-sparteine in the laboratory using only catalysts for the synthesis! If you are truly to emulate Nature, then you would not be allowed to use protecting groups. Moreover, imagine being given an exam question in which you are asked to generate a synthesis of (−)-sparteine using cadaverine as the starting material!
Figure 1.3 Biosynthetic pathway for the conversion of cadaverine to (−)-sparteine.
However, despite the abundance of natural products and biosynthetic pathways in Nature, the enzymes involved in secondary metabolism have not found their way in a general sense into the organic chemist's toolbox. There are a number of reasons for this situation. Firstly, enzymes of secondary metabolism often have relatively low activity, probably as a consequence of the fact that natural products are often produced in low concentrations and hence there is no need for enzymes with high catalytic turnover. Secondly, these enzymes are often quite specific for particular intermediates along the biosynthetic pathway and hence do not lend themselves to being applied in a general sense to a broader range of substrates. Biosynthetic enzymes have, however, provided an enormously rich playground of molecules and associated enzymes in order to gain fundamental understanding of the enzyme mechanism and the ways in which complex molecules can be assembled.
This book is about exploring a ‘third’ approach to organic synthesis, namely the use of engineered biocatalysts. Unlike natural product biosynthesis, these enzymes will predominantly be derived from primary metabolism, and hence should have inherently higher catalytic rates since their natural function is to process large quantities of building blocks and intermediates that are essential to cellular metabolism (e.g. amino acids, carbohydrates, and nucleotides). The challenge here is to develop an increasingly broader range of distinct biocatalysts such that a wider range of organic molecules come into play and become potential targe...