
eBook - ePub
Membrane Engineering for the Treatment of Gases
Volume 2: Gas-separation Issues Combined with Membrane Reactors
- 366 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Membrane Engineering for the Treatment of Gases
Volume 2: Gas-separation Issues Combined with Membrane Reactors
About this book
Elaborating on recent and future developments in the field of membrane engineering, Volume 2 is devoted to the main advances in gaseous phase membrane reactors and separators. The book covers innovative membranes and new processes, and includes new chapters on cost analysis and life cycle assessment. Together with Volume 1, these books form an innovative reference work on membrane engineering and technology in the field of gas separation and gaseous phase membrane reactors.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Membrane Engineering for the Treatment of Gases by Enrico Drioli, Giuseppe Barbieri, Adele Brunetti in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Industrial & Technical Chemistry. We have over one million books available in our catalogue for you to explore.
Information
CHAPTER 1
Membrane Reactors for Hydrogen Production
a Institute on Membrane Technology (ITM-CNR), National Research Council, c/o The University of Calabria, Cubo 17C, Via Pietro Bucci, 87036 Rende CS, Italy
b Department of Environmental and Chemical Engineering (DIATIC) The University of Calabria, Cubo 44A, Via Pietro Bucci, 87036 Rende CS, Italy
*Email: [email protected]
1.1 Introduction
In the last decade, the energy demand has grown by 1.2% a year and fossil fuels still maintain a production share of ca. 75%. However, the ever stricter problems connected to sustainable growth and lower environmental impact lead to the conclusion that the times of easy oil consumption are over. Nowadays, the necessity to produce energy from oil and natural gas as primary energy sources is becoming more and more pressing. Indeed, more generally, the diversification of said sources in order to ensure a constant supply makes the interest in membrane reactor (MR) technology more urgent. Moreover, the increasing efforts dedicated to the reduction of environmental problems has recently led to the development of clean technologies, designed to enhance both the efficiency and environmental acceptability of energy production, storage, and use, in particular for power generation.1 Among these technologies, the exploitation of light hydrocarbons is surely the main realistic energy source, since they allow both power generation and environmentally friendly fuel production. Specific reference should be made to hydrogen in this context.
At present, the global hydrogen production relies mainly on processes that extract hydrogen from fossil fuel feedstocks. About 96% of hydrogen is directly produced from fossil fuels and about 4% is produced indirectly using electricity generated through them.2 The stream coming out from a reformer or a coal gasification plant contains around 50% hydrogen (on a dry basis) that must be recovered and between 40–45% CO that is usually reduced in an upgrading stage, producing more hydrogen at the same time. In traditional applications (Figure 1.1), the upgrading of reformate streams is performed using a multi-stage CO-shift process based on a series of catalytic reactors: the first one operates at high temperatures (about 350–400 °C) and takes advantage of the high reaction rate, converting a large portion of CO into hydrogen and CO2; the other one operates at lower temperature (around 220–300 °C) and refines the carbon monoxide conversion, thus allowing a lower final concentration of CO (less than 1% molar).3 This H2-rich stream coming out from the last reactor is fed to a pressure swing adsorption (PSA) unit for H2 separation from other gases. It should be pointed out that the new utilization of H2 as feed in fuel cells for mobile power sources requires the anode inlet gas to have a CO concentration below 10–20 ppm4 in order to avoid catalyst poisoning with subsequent drops in fuel cell efficiency. Hence, the purification step for the H2 produced from hydrocarbons must be very efficient to fulfil said fuel cell requirements. Because of this, in some cases, another reaction unit is added to oxidize CO into CO2.
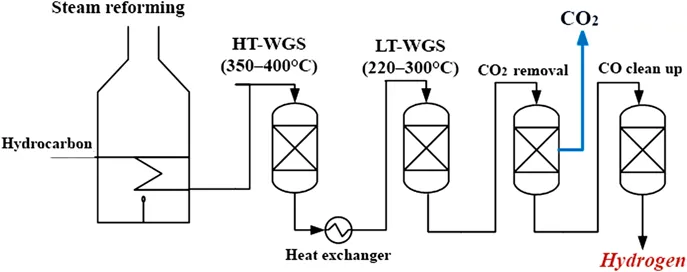
Figure 1.1 Scheme of the traditional process for hydrogen production from light hydrocarbons. Reproduced from ref. 105 with permission from the Royal Society of Chemistry.
One of the main challenges in the next few years will be the identification of new technologies able to provide better exploitation of fossil fuels, e.g., hydrocarbons, in order to improve the yield, energy savings, and so on. The reduction of the number of reaction/separation/purification stages, which translates into a lower footprint area occupied by the whole plant, fewer auxiliary devices, reduction of the energetic load, and so forth, is a fundamental issue to consider when redesigning hydrogen production processes. A promising approach for concretizing these technological aspects in the field of hydrogen production is the use of MRs, combining the reaction and H2 separation by means of selective membranes. Many studies are now focused on the analysis of MR performance, where light hydrocarbon reforming or water–gas shift (WGS) reactions are carried out. In these cases, for both reactions, the presence of a membrane allows the recovery of a hydrogen-rich stream that does not require further separation/purification. Moreover, the removal of H2, the reaction product, from the reaction volume shifts the reaction toward further conversion. This means the possibility of having an intensified process with a reduced plant size and higher yield. The traditional process can thus be redesigned in a more compact and efficient manner (Figure 1.2), following the logic of the Process Intensification Strategy, which is an innovative methodology for process and plant design proposing a new design philosophy to achieve significant reductions (by factors of 10 to 100 or more) in plant volume at the same production capacity or to improve the overall efficiency.
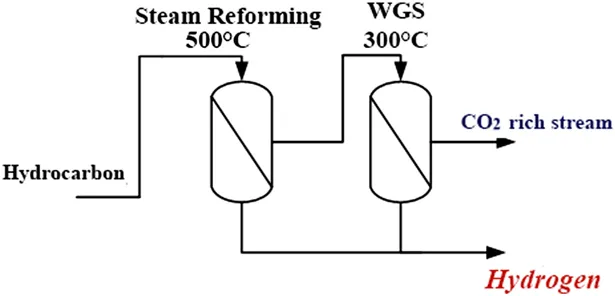
Figure 1.2 Scheme of an integrated membrane process for hydrogen production from light hydrocarbons. Reproduced from ref. 105 with permission from the Royal Society of Chemistry.
Figure 1.2 shows an integrated membrane system constituted by fewer reaction/separation units than the conventional one (Figure 1.1). A first MR can be used to carry out the reforming of light hydrocarbons and another reactor for the WGS reaction.
The presence of a membrane in both reactors allows the separation of a hydrogen-rich stream from the two reaction volumes, as well as improvements in the conversion of the two stages. Obviously, the H2 purity level strictly depends on the membrane type used in each MR. In fact, membranes can be distinguished by their selectivity, which can be infinite or finite. The first ones, traditionally Pd-based, allow a pure hydrogen stream to be obtained, whereas the others provide a hydrogen-rich stream of variable purity. If the recovered H2 stream does not have the purity required, the latter can be increased by adding another purification unit depending on the final use of the H2 stream. Selective CO oxidation is known as an interesting and economical approach for CO removal from H2-rich gas streams. Also in this field, new studies proposed in the literature have demonstrated how the use of MRs can improve the process by increasing the CO conversion as well as the purity of the hydrogen stream.
In this context, membrane engineering plays a fundamental role in the integration of these units into a single plant and, at the same time, in the definition of the knowledge necessary to drive the process by maximizing the gains, both in terms of efficiency and plant size reduction. The synergic effects offered by MRs by combining reaction and separation processes in the same unit, their simplicity, and the possibility of advanced levels of automation and control offer an attractive opportunity to redesign industrial processes.5–8
1.2 Membranes for Hydrogen Production
MRs represent the most significant class of the so-called multifunctional reactors,9 which integrate reaction and separation processes in the same unit. Membranes for hydrogen separation should exhibit high selectivity toward hydrogen and high flux, being in the meantime highly mechanically and chemically stable. These aspects are very well addressed in Chapter 4 of this volume.
Most of the membranes used in hydrogen production allow the selective removal of H2 from the reaction volume under the effect of a driving force. This is a function of the species partial pressure on each membrane side and can be creat...
Table of contents
- Cover
- Title
- Copyright
- Preface
- Contents
- Chapter 1 Membrane Reactors for Hydrogen Production
- Chapter 2 Chemical Looping for Hydrogen Production and Purification
- Chapter 3 Oxidative Coupling of Methane in Membrane Reactors
- Chapter 4 Ultrathin and Thin Film Pd/Ag Membranes for Hydrogen Production
- Chapter 5 Polarization and CO-inhibition in Pd-based Membranes and Membrane Reactors
- Chapter 6 Pd-based Membranes in Hydrogen Production: Long-term Stability and Contaminant Effects
- Chapter 7 Membrane Processes for Pure Hydrogen Production from Biomass
- Chapter 8 Membrane-assisted Syngas Production for Gas-to-Liquid Processes
- Chapter 9 Mixed Ionic–Electronic Conducting Membranes for Hydrogen Separation
- Chapter 10 Inorganic Membranes for Gas Separation
- Subject Index