1.1 Background
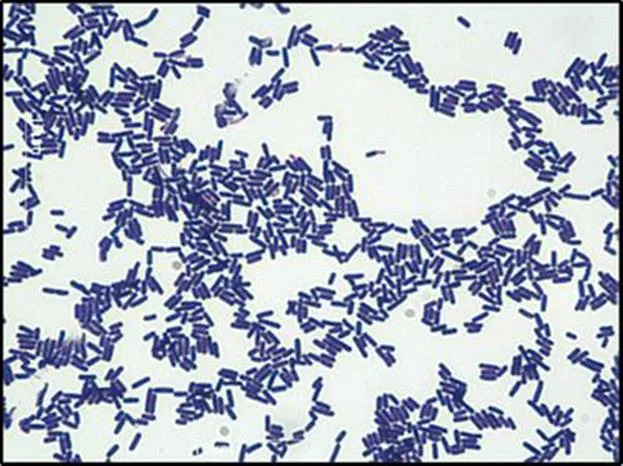
Clostridium difficile infection (CDI) is a nosocomial disease mainly correlated with antibiotic-associated diarrhea. These infections are caused by Clostridium difficile, an anaerobic, rod shaped, gram-positive bacterium that is normally found in the gastrointestinal tract (Figure 1.1).1
Figure 1.1 Gram stain of C. difficile. Vegetative C. difficile cells are rod shaped and stain purple in the presence of crystal violet, displaying a gram-positive phenotype. Scale = 10 µm.
Approximately 5% of healthy adults, and 50% of newborns are asymptomatic carriers of C. difficile.2 C. difficile was originally thought to be a commensal bacterium, but due to the recent boom of antibiotic therapies and advancements, it was quickly recognized that C. difficile is the leading cause of hospital-acquired diarrhea worldwide.3 In the United States alone, there are roughly 500 000 cases of CDI annually, with associated costs estimated to be approximately $4.8 billion.4
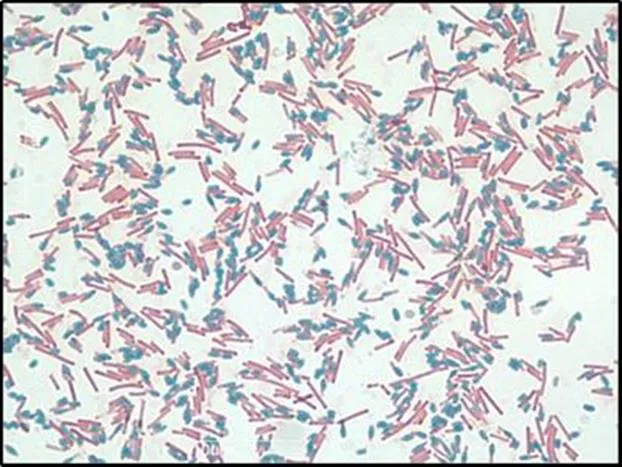
C. difficile has a unique lifecycle such that it can form metabolically dormant, non-reproductive spores when stressed (Figure 1.2).5
Figure 1.2 Schaeffer-Fulton stain of C. difficile. Upon environmental stress and starvation, vegetative C. difficile cells (pink) begin to produce highly resistant, non-reproductive spores (blue). Scale = 10 µm.
These stressors include, but not limited to, nutrient limitation and desiccation. The resulting spores are highly resistant to harsh environmental factors such as stomach acid, extreme temperatures, and pharmaceutically relevant antibiotics.6 Spores can persist over prolonged periods of time, while constantly monitoring the environment for favorable conditions. Upon reintroduction to nutrient rich environments, the spores are able to revert back into actively growing cells through a process known as germination.6 When C. difficile has completed its lifecycle, transitioning from a spore to an actively growing cell, the newly germinated cells can now colonize the local environment.
CDI begins with the ingestion of the highly resistant C. difficile spores. As these spores travel through the gastrointestinal tract, various endogenous bile salts stimulate the spores to germinate into actively growing, vegetative cells.7 While the spores are metabolically dormant and act solely as a vehicle for infection, the vegetative cells are metabolically active, can produce toxins, and elicit disease.
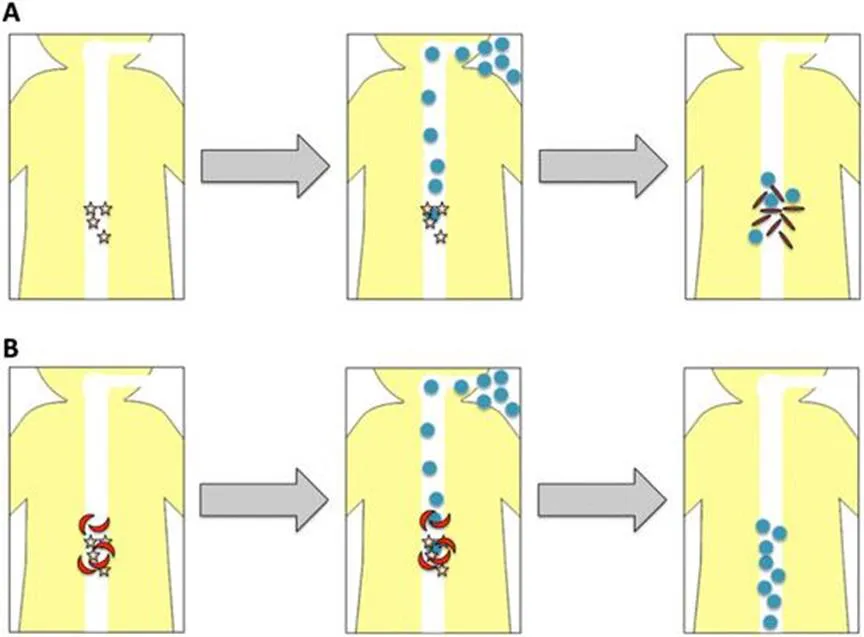
The diversity of the endogenous bile salts depends heavily on the intestinal gut flora. Under normal circumstances, the bacteria found naturally in the gastrointestinal tract provide a barrier against C. difficile colonization by occupying nutrient-rich niches and by metabolizing specific bile salts required for C. difficile germination.8 With the current pharmaceutical advancements, several new broad-spectrum antibiotics have been developed. Exposure to antibiotics, such as clindamycin, 2nd and 3rd generation cephalosporins, and fluoroquinolones, can disrupt the natural gut flora.2,9,10 Disruption of the gut flora effectively removes the protective barrier, as the change in the bile salt diversity becomes more favorable for C. difficile (Figure 1.3).
As C. difficile spores germinate and outgrow, the resulting vegetative cells begin to produce and release two major toxins, TcdA and TcdB. The C-terminal region of both TcdA and TcdB contain a binding domain, which is able to interact with different carbohydrate and protein structures found on the surface of host cell membranes.11 TcdB binds to chondroitin sulfate proteoglycan 4 (CSPG4) and the poliovirus receptor-like 3 (PVRL3) found on the surface of intestinal epithelial cells.12 In contrast, TcdA can bind to glycoprotein 96 and sucrase isomaltase, both of which are found on the surface of human colonocytes. Once these toxins interact with the host cell receptors, the toxins are internalized by endocytosis. Acidification of the endosome causes the toxins to undergo conformational changes resulting in translocation into the host cell cytoplasm. Upon entry into the cytoplasm, the toxins undergo autocatalytic cleavage.11 Cleavage of the toxins allow for the release and activation of their glycosyltransferase domain (GTD) into the host cell.
Figure 1.3 (a) Germination of C. difficile spores and the use of anti-germinants. Upon antibiotic therapy the diversity of bile salts (yellow stars) shift to a population more favorable for C. difficile spore germination. As C. difficile spores (blue circles) are ingested, the spores travel through the gastrointestinal tract, recognize the specific bile salts, and are able to germinate into toxin producing cells (red rods). (b) In the presence of various bile salt analogs (red moons), as C. difficile spores travel through the gastrointestinal tract, the bile salt analogs compete with bile salts that would normally trigger germination, effectively blocking C. difficile germination and the progression of CDI.
The GTD can transfer glucose from UDP-glucose to several crucial Rho proteins.11,13 Glucosylation of Rho proteins result in their inactivation.11 Since Rho proteins play an essential role in regulating the cell cytoskeleton, inactivation of these proteins can have several cytopathic effects including the disruption of cell-to-cell contacts and tight junctions, as well as increased epithelial permeability.14 Glucosylation of RhoA also activates the inflammasome and upregulates a pro-apoptotic protein, RhoB.
Expression of tcdA and tcdB is heavily regulated and dependent on resource availability. When carbon sources and other nutrients are readily available, toxin expression is inhibited.11 Conversely, toxin production is upregulated during stationary phase when resources are low. This type of regulation suggests that C. difficile virulence is a killing strategy used to improve resource availability by scavenging the host cell for resources. A combination of several factors contributes to C. difficile’s virulence. TcdA and TcdB are undoubtedly major contributors.11 Due to genetic variability between C. difficile strains, the extensive genotypic variances in the pathogenicity locus (PaLoc), which houses tcdA and tcdB, currently give rise to at least 31 different toxinotypes.11,15 These different toxinotypes result from mutations in tcdA and tcdB, as well as the regulatory factors that ultimately lead to the overexpression or repression of the toxin genes.15 Several other factors, such as rates of sporulation and toxin release, motility and host cell adherence, can contribute to C. difficile virulence.
Interestingly, so called “hypervirulent” strains of C. difficile have begun to reveal themselves in the healthcare setting worldwide. Hypervirulent strains are highly variable—some strains may have higher rates of sporulation and toxin production.13 TcdC, an anti-sigma factor that acts as a negative regulator of toxin production, is upregulated during exponential growth.15 Several hypervirulent strains contain mutations in tcdC that result in the constant, unregulated production of C. difficile toxins.15 Toxins produced by hypervirulent strains can also undergo necessary conformational changes at higher pH ranges, allowing the toxins to enter the host cell cytoplasm earlier and at a faster rate, relative to toxins produced by non-hypervirulent strains.11 Clostridium difficile transferase (CDT), a ribosyltransferase, is a binary toxin produced only by several strains of C. difficile. Similar to TcdA and TcdB, CDT binds to a host-cell by interacting with a surface molecule, specifically lipolysis-stimulated lipoprotein receptor (LSR).16,17 CDT eventually localizes into the host cell cytoplasm where it begins to ribosylate G actin.15 At low concentrations of the toxin, CDT induces microtubules to form protrusions from the host-cell membrane, facilitating C. difficile adherence to the surface of the intestinal epithelial cells.15,18 At high concentrations of CDT, actin polymerization is inhibited and actin depolymerization is induced ultimately causing the collapse of the host cell cytoskeleton.17