
eBook - ePub
Sustainable Synthesis of Pharmaceuticals
Using Transition Metal Complexes as Catalysts
- 286 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Sustainable Synthesis of Pharmaceuticals
Using Transition Metal Complexes as Catalysts
About this book
There is a growing interest in the development of sustainable processes for the synthesis of pharmaceuticals and this book bridges the divide between industrial examples and the fundamental chemistry. It explains the basic principles of using transition metal catalysis with several green approaches for the synthesis of pharmaceuticals. The topic is an important one for green chemistry and the chapters in this book on hydroformylation, green oxidation and olefin metathesis will also be of interest to both medicinal and organic chemists.
Written by leading experts in the field, it provides a valuable and easy tool for scientists and industrialists who require information regarding this topic.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Sustainable Synthesis of Pharmaceuticals by Mariette M Pereira, Mário J F Calvete in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Environmental Science. We have over one million books available in our catalogue for you to explore.
Information
CHAPTER 1
Introduction
University of Coimbra, CQC, Department of Chemistry Rua Larga Coimbra 3004-535 Portugal
*Email: [email protected]; [email protected]
*Email: [email protected]; [email protected]
1.1 Introduction
The development of sustainable processes for the synthesis of new active pharmaceutical ingredients (API) continues to be one of the great challenges for medicinal chemistry at universities and in the pharmaceutical industry.1
Given the benefits to public health, for decades, the pharmaceutical industry was more concerned with the end product than with the means of producing it. For decades, the synthetic methods of pharmaceutical products were the ones that led to greater waste and those that least respected the principles of green chemistry (green chemistry preferentially utilizes raw materials, avoids toxic and hazardous reagents and solvents, eliminates waste and when possible reduces the energy consumption, Chapter 3). However, in the last decade several companies have decided to adhere to the philosophy of green chemistry by modifying their production processes and especially by designing new processes where metrics (Chapter 3) have already been taken into account and the principles of waste and solvent reduction have been considered very important issues for the development of new API process development.
A key feature for improving the sustainability of the pharmaceutical industry is the design of new processes according to the principles of green chemistry. In the last few decades several companies changed their practices and adhered in particular to principle number 9: “catalytic reagents (as selective as possible) are superior to stoichiometric reagents”. Nowadays, it has become clear to the pharmaceutical industry that the substitution of stoichiometric chemical reactions by catalytic processes may solve several industrial problems: (i) increasing selectivity for the desired product, particularly as regards the synthesis of enantiomerically pure pharmaceuticals; (ii) reducing the costs due to lower energy consumption; and (iii) reducing solvents and the overall process cost.
The great relevance of the use of organometallic reagents as catalysts for organic synthesis is clearly evidenced by the attribution of several Nobel Prizes in the field.2
This book is aimed at researchers or post-graduate students, both in academia and the pharmaceutical industry, who are interested in developing processes for synthesis of drug synthons or APIs, using transition metals catalysts as the tool for achieving green chemistry purposes. As Clark reports in Chapter 2, the substitution of toxic metals by less toxic ones like iron and the development of new processes for recovering catalysts is clearly a new paradigm that the pharmaceutical industry should consider when introducing metals as catalysts for the development of new drugs. This issue is focused on in the great majority of the chapters.
In Chapter 2, J. Clark elucidates on the availability of critical chemical elements, mostly metals, and the strong discouragement of their use since there is a growing appreciation that not only are resources limited, but also their recovery is very difficult, and this vital part of sustainability must be recognized within green chemistry. It is crucial that efforts are increased both to use less metals and to design catalysts and processes to maximize recovery of the metals. Heterogeneous catalysts can definitely play a major role in this endeavour.
In Chapter 3, M. Pineiro and M. Calvete discuss the success of the philosophy and principles of green chemistry in the active search for more sustainable drug synthesis processes. A collection of diverse approaches has been reported so far, including the use of alternative reaction media and alternative technologies, such as microwaves, mechanochemistry, and ultrasound, especially when combined with new catalysts and catalytic systems, where the sustainability “improvement” is measured and quantified by using green chemistry metrics integrated in the drug discovery and development process in the pharmaceutical industry.
In Chapter 4, R. Skoda-Földes and L. Kollár shed light on pharmaceuticals arising from one-pot carbonylation processes, as typically the production of pharmaceuticals usually involves multistep syntheses where the selectivity and yield of the individual steps are of utmost importance. Among highly efficient catalytic processes, carbonylation received special attention as it involves both new carbon–carbon bond formation and the introduction of a synthetically useful functionality in the synthesis of carbonyl compounds and carboxylic acid derivatives. To achieve widespread application, more efficient catalysts should be developed that ensure higher turnover numbers and make it possible to carry out carbonylations at atmospheric conditions as well as to replace the starting material aryl iodides with cheaper bromides or, still preferably, chlorides. This reaction was described as a greener approach to prepare pharmaceuticals or their precursors bearing carboxylic acids, amides or esters functionalities in just one-pot.
In Chapter 5, M. Pereira highlights several aspects concerning the mechanism of rhodium-catalysed hydroformylation (still considered the “metal of choice” owing to its high activity and selectivity), the development and evolution of new metal catalysts and phosphorus ligands from a historical perspective, some strategies related to the synthesis of reusable catalysts for use in alternative media and the utilization of less toxic solvents and alternative metals. A set of selected examples for the direct transformations of olefins into aldehydes, via one-step 100% atom economy process, for the sustainable preparation of pharmaceutical intermediates or APIs is also described.
In Chapter 6, B. Royo describes the application of metal-catalysed asymmetric transfer hydrogenation and borrowing hydrogen processes to the synthesis of pharmaceuticals, especially those using Earth-abundant catalysts, which can replace precious metals, enabling simple and safe synthetic strategies with high atom economy and efficiency, providing an outstanding input for the discovery of new bioactive molecules useful in the pharmaceutical industry.
In Chapter 7, C. Romão discusses the development of green metal asymmetric epoxidation and sulfoxidation catalysis, obeying the highest possible number of “green chemistry” rules, and exploring/expanding the control over the exquisite mechanisms of both epoxidation and sulfoxidation, posing a very high research target that could lead to waste-free fully sustainable oxidation processes. Their very existence proves the endeavour is worth pursuing.
In Chapter 8, L. Martins, A. Phillips and A. Pombeiro provide a discussion on C–C bond formation in the sustainable synthesis of pharmaceuticals (APIs or other drug components), whose current production includes transition metal-catalysed C–C cross coupling reactions as key steps of the synthetic processes in view of their mildness, functional group compatibility and the high turnover of used catalysts. Emphasis is put on modern protocols, which allow the purge of metal catalysts, whilst still providing high purity compounds, by continuing development of optimized catalysts, ligands, additives and reaction conditions in a green environment.
In Chapter 9, E dos Santos, A. Granato, and A. Santos elucidate on metal-catalysed olefin metathesis reactions for greener synthon/drug synthesis, providing detailed valuable information on catalysts and conditions to perform metathesis reactions. Various applications of metathesis in the synthesis of active pharmaceutical ingredients have been recently disclosed. It has also been very important for chemical diversity generation and drug discovery, giving rise to new applications in the industrial synthesis of pharmaceuticals.
In Chapter 10, Q. Meng, M. Wang, and M. Vicente highlight tetravalent boron-based therapeutics, particularly with anticancer, antiviral, antibacterial and antifungal activities. The number of boron-containing therapeutics is expected to continue to increase as new synthetic compounds, such as those based on BODIPYs and related systems, continue to be investigated for potential applications in diagnosis, and in the treatment of cancer and other diseases, including via photodynamic and boron-neutron capture therapies.
References
1.J. Verghese, C. J. Kong, D. Rivalti, E. C. Yu, R. Krack, J. Alcázar, J. B. Manley, D. T. McQuade, S. Ahmad, K. Belecki and B. F. Gupton, Green Chem., 2017, 19, 2986.
2.A. M. Thayer, Chem. Eng. News, 2013, 91, 68.
CHAPTER 2
Transition Metals in Greener Pharmaceutical Chemistry
Green Chemistry Center of Excellence, University of York, York YO105DD, United Kingdom
Email: [email protected]
Email: [email protected]
2.1 Transition Metals in Greener Pharmaceutical Chemistry
When the first concerns about the future availability of some critical elements—mostly metals—were being discussed in Europe, there was a school of thought that Brussels might become a “metal-free zone”, in other words that the EU would try to discourage the use of (some) metals in (some) applications. The growing appreciation that we cannot continue to use resources as though they were unlimited, and in a way that makes their recovery very difficult, is a vital part of sustainability and must be recognized within green chemistry. Medium-term availability problems are made worse by the rapid growth of new technologies that are ironically often driven by the desire for low-carbon energy, which use elements at levels never previously seen. This includes wind turbines (which use dysprosium, neodymium and praseodymium) and electric vehicles (antimony, neodymium, dysprosium and gadolinium) as well as rapid developments in batteries, which are using more and more lithium.
The latest and more holistic green chemistry metrics for pharmaceutical (and other fine chemical) processes accommodate this new concern as one of the key impacts to take into consideration (Figure 2.1).1
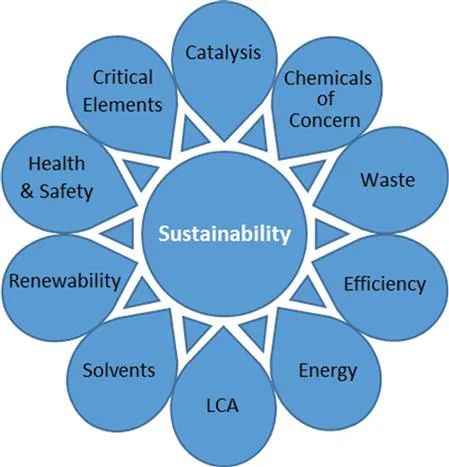
Figure 2.1 Critical impacts to be considered for all processes. LCA stands for Life Cycle Assessment. Reproduced from ref. 1 with permission from The Royal Society of Chemistry.
In the associated online tool that assesses the green credentials of any new or existing process, a red flag is waved over any metal used that is considered to be “critical” although such classifications can change as new technologies grow while others fade and as a result of a new discovery of the associated ore. New ore discoveries will however, almost certainly come at higher economic and environmental cost—we have exploited most of the easy resources.
It would, however, be oversimplified and unwise to seek to avoid the use of all critical metals as catalytic materials. The benefits may outweigh concerns over the availability of some metals, at least until we find adequate replacements. Catalysis continues to be the most important green chemical technology and is of course one of the key impacts in Figure 2.1. Metals play a dominant role in catalysis. Palladium, for example, has become a highly valued catalyst in many cross-coupling reactions, including Heck, Negishi and Suzuki, reactions that are increasingly used in the manufacture of pharmaceuticals. Alternative Pd-free routes, where possible, are generally more laborious, involve several additional reagents and create substantial quantities of waste. Palladium is currently considered to be of medium availability concern, with known reserves and current rate of consumption giving 50–100 remaining years, although this could easily change either way. Other very useful catalytic metals including ruthenium and iridium are believed to be down to less than 50 years, making their sustainability of more immediate concern and their continued use may be more problematic.2
In all cases it is important that we increase our efforts both to use less metal and to design the catalyst and process to maximize recovery of the metal. Heterogeneous catalysts can make recovery easier as may other two-phase reaction systems [e.g. where the catalyst and substrate(s) are in different liquid phases] but there is always a concern about leaching from the catalyst phase, likely leading to loss of the metal and to possible contamination of the product (which is strictly controlled for pharmaceutical compounds). We do need to develop new technologies for recovering trace metals from product mixtures as well as from wastes more generally. As we become more concerned about the sustainability of many metals and some other important elements (e.g. phosphorus) we should seriously consider new sources of those elements that are now in the form of wastes: WEEE (Waste Electrical and Electronic Equipment), mine tailings, emissions from catalytic convertors and others. Here techniques including phytomining (the recovery of metals from land using plants) might have an important role.3
Of course, an important alternative strategy to finding solutions to the continued use of critic...
Table of contents
- Cover
- Title
- Copyright
- Contents
- Chapter 1 Introduction
- Chapter 2 Transition Metals in Greener Pharmaceutical Chemistry
- Chapter 3 Sustainable Synthesis of Pharmaceuticals Using Alternative Techniques: Microwave, Sonochemistry and Mechanochemistry
- Chapter 4 Carbonylation Reactions in the Synthesis of Pharmaceutically Active Compounds
- Chapter 5 Applications of Catalytic Hydroformylation in the Synthesis of Biologically Relevant Synthons and Drugs
- Chapter 6 Transfer Hydrogenation with Non-toxic Metals for Drug Synthesis
- Chapter 7 Green Metal-catalysed Synthesis of Pharmaceutically Useful Asymmetric Epoxides and Sulfoxides
- Chapter 8 C–C Bond Formation in the Sustainable Synthesis of Pharmaceuticals
- Chapter 9 Metal-catalysed Metathesis Reactions for Greener Synthon/Drug Synthesis
- Chapter 10 Tetravalent Boron-based Therapeutics
- Subject Index