![]()
CHAPTER 1
Polymerization of Phosphorus-Containing (Meth)acrylate Monomers
SOPHIE MONGE,* BENJAMIN CANNICCIONI, GHISLAIN DAVID AND JEAN-JACQUES ROBIN
Institut Charles Gerhardt de Montpellier, UMR5253 CNRS-UM2-ENSCM-UM1, Equipe Ingénierie et Architectures Macromoléculaires, Université
Montpellier II, cc1702, Place Eugène Bataillon, 34095, Montpellier, France
*Email:
[email protected]1.1 Introduction
In recent years, phosphorus-based polymers have been widely studied1–6 as they exhibit very unusual and interesting properties.7 Whereas the ester forms are the most available compounds, monoacids and diacids can be easily obtained under mild conditions with the use of bromotrimethylsilane, opening the way to a wide range of polymers showing different properties. The latter can be explained in part by the ionization potential of phosphonic acids, which is intermediate between that of sulfonic and carboxylic acids due to their intermediate pKa.
Phosphorus-containing materials can be employed for a wide range of technological applications.8 For instance, they are extensively used in industry, notably to bind metals.9–12 Indeed, phosphorus-based materials show interesting complexing properties13,14 and are used as dispersants, corrosion inhibiting agents, or for preventing deposit formation.15 They are also involved in flame retardancy,16,17 where phosphorus is known to be particularly useful. An important industrial application deals with their use in the biomedical field,18 as they are biodegradable, blood compatible, show reduced protein adsorption, and lead to strong interactions with dentin, enamel, or bones. As a consequence, various syntheses of monomers and polymers are carried out following different procedures: (i) introduction of the phosphorinated moieties onto polymers by (co)polymerization of monomers bearing the phosphorus atom or (ii) grafting of phosphorus-based groups onto the polymer.
In this contribution, we will focus on the polymerization of phosphorus-based (meth)acrylates. Different kinds of monomers will be considered, as a function of the targeted applications. The latter will be more thoroughly discussed in the second part of this book (Chapters 8–13). The resulting phosphorus-based poly(meth)acrylates are mainly used for anticorrosion, flame retardancy, tissue engineering, and dental applications. Concerning anticorrosion, polymers have been involved in corrosion protective coatings which require maintenance of adhesion under environmental exposure. Adhesion between galvanized steel plates and the polymer depends on the chemical structures of both substrate and coating and reduction of adhesion causes water penetration at the coating/metal interface, leading to a significant reduction of adhesion. Incorporation of phosphonic groups, known as adhesion promoters, into polymer structures allows improvement of the adhesion properties of polymers on metallic surfaces.19 Furthermore, research has also been carried out on the development of new halogen-free flame retardants. In this context, phosphorus is known to be efficient with or without other elements like nitrogen or sulfur. Concerning tissue engineering, polymeric materials, especially phosphonated polymers, have already proved to be of great interest. Tissue engineering typically involves the seeding of biodegradable polymeric scaffolds with differentiated or pluripotent cells in vitro, followed by implantation of the whole cell–scaffold system into the region of tissue loss or damage. It has been shown that protein interactions were favored when phosphorus-grafted polymeric surfaces were used. Finally, among all self-etching adhesive systems developed in dentistry for bonding of resin composite to enamel or dentin, primers containing phosphonated or phosphonic acid groups have been quite widely considered. Indeed, such derivatives are potentially interesting as the incorporation of a phosphonic function would result in an increase of the biocompatibility and in the adhesion due to chelation with calcium ions at the tooth surface20 because of complex formation with calcium in hydroxyapatite.21 This mineral is partially dissociated by phosphonic acid22 to give brushite acting as a macromolecular crosslinker23 and the bond strength depends on the alkyl chain length of the monomer.24,25 For this last application, bis(meth)acrylate monomers are also considered. Finally, special attention will be paid to the controlled radical polymerization of dimethyl [(methacryloyloxy)methyl]phosphonate, very recently reported in the literature, demonstrating that it is possible to prepare well-controlled architectures involving phosphorus-based monomers.
1.2 Synthesis by Free or Controlled Radical Polymerization of Phosphorus-Based Poly(meth)acrylates as Adhesion Promoters
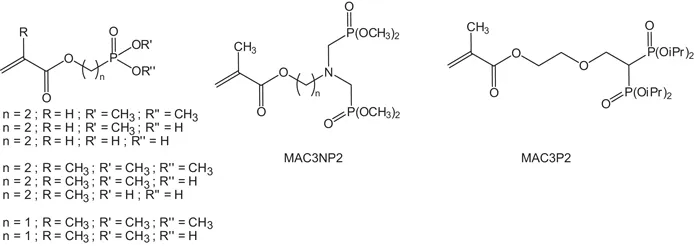
Free-radical graft copolymerization of phosphonated (meth)acrylates onto polymers is carried out with dimethyl [2-(meth)acryloxyethyl]phosphonate (DAP) or corresponding mono- or diacid derivatives (DAP monoacid or DAP diacid) (Scheme 1.1).26 These monomers are obtained using a simple synthetic method starting from commercial compounds. DAP is obtained by an esterification reaction involving acryloyl chloride and dimethyl (2-hydroxyethyl)phosphonate in dichloromethane in the presence of triethylamine. The synthesis of phosphonic acid monomer derivatives (DAP monoacid and DAP diacid) is achieved in two steps: (i) silylation of a dimethyl phosphonate function by bromotrimethylsilane, followed by (ii) hydrolysis with an excess of methyl alcohol. Using an equivalent amount of brominated silane and DAP leads to a mixture of three functional phosphonated groups (notably with DAP monoacid), whereas an excess of bromotrimethylsilane quantitatively converts phosphonated moieties into phosphonic diacid groups (DAP diacid). Then, ozone-oxidized poly(vinylidene difluoride) (PVDF) is grafted with phosphonated acrylate monomers and acrylic acid by two methods: solution or bulk polymerization. Polymerization in solution is achieved using dimethylformamide (DMF) as solvent at 90 °C for 24 h, or deionized water at 60 °C for 12 h. For bulk polymerization, the reaction is carried out at 130 °C in an air atmosphere for the prescribed time. Then, the graft copolymer is dissolved in DMF and the homopolymer is removed by precipitation in ethyl alcohol. Adhesion of the graft copolymers was subsequently studied. The presence of phosphonic acid groups leads to a significant improvement in the adhesive bond to metal compared to both dialkyl phosphonate and carboxylic acid groups. The same kinds of experiment were carried out with low-density polyethylene powders27 using phosphonated methacrylates by grafting in the presence of free-radical initiators or thermally induced graft copolymerization onto ozone-pretreated low-density polyethylene.
Scheme 1.1 Phosphorus-based (meth)acrylate monomers used for adhesion properties.
Poly(MMA)-b-poly(monophosphonic methacrylate) (MMA=methyl methacrylate) diblock copolymers are also prepared by atom transfer radical polymerization (ATRP) and used as additives in PVDF coatings to protect steel against corrosion.28 ATRP of dimethyl [(methacryloyloxy)methyl]phosphonate (MAPC1), prepared by the Pudovic reaction,29 was investigated in toluene, in the presence of methyl 2-bromoisobutyrate as the initiator, and using different metal and ligand systems. Polymerization proceeded with very low monomer conversion, which was attributed to the ability of phosphorus to complex the copper ions, removing copper ions from the original ligand stopping the MAPC1 polymerization. As a result, another strategy was chosen for an efficient synthesis of poly(MMA)-b-poly(phosphonate acrylate) diblock copolymer (without phosphonated-based monomer), which was efficiently obtained by a four-step reaction, with first the synthesis of poly(MMA)-b-poly(tert-butyl acrylate) diblock copolymer by ATRP. Then the tert-butyl groups were removed and phosphonate functions were incorporated by esterification. This new diblock copolymer was used as an additive for an anticorrosive coating, but no improvement (using the salt spray test technique) was observed compared with the statistical copolymer with the same acid content.
Blends of phosphorus-containing copolymers with PVDF powders were also investigated to enhance adhesive and anticorrosive properties of fluoro polymer coatings.30 Statistical copolymers were first prepared copolymerizing MMA and dimethyl [(2-methacryloyloxy)ethyl]phosphonate, [(2-methacryloyloxy)ethyl]phosphonic diacid, or methyl [(2-methacryloyloxy)ethyl]phosphonic hemiacid. Concerning the synthesis of the monomers involved, dimethyl (2-hydroxyethyl)phosphonate reacted with methacryloyl chloride in the presence of triethylamine in dichloromethane. The resulting dimethyl [(2-methacryloyloxy)ethyl]phosphonate was isolated by distillation under vacuum (2×10−3 mbar) at 85 °C. The acid derivatives were synthesized by methanolysis using bromotrimethylsilane and methanol. Copolymerizations were then carried out at 70 °C either in THF or DMF in the presence of AIBN. The resulting copolymers were introduced into PVDF as adhesion promoters and anticorrosion inhibitors. Good dry ...