Milk and methanol have little in common other than that they are both important to society and that both have been subjected to processes designed and operated by chemical engineers.
The milk that the consumer buys in the store has been subjected to at least three main processes: pasteurisation, homogenisation and standardisation. Each of these processes has been carefully designed to meet a different but specific need – pasteurisation kills potentially dangerous micro-organisms in the milk using heat, homogenisation breaks down fat globules preventing the milk from separating into different layers, and standardisation ensures that the milk is always of a specific fat content no matter what the fat content of the original milk. Chemical engineers ensure that these processes operate efficiently, safely and economically, while bearing in mind that the milk must always be fit for human consumption at the end of the process.
Methanol is an example of an industrially important chemical that is manufactured worldwide on a large scale. Methanol, sometimes known as wood alcohol, is used as a racing fuel in some types of car and truck racing. It is more important role, however, is as a feedstock in the production of a range of other chemicals such as formaldehyde and dimethyl ether. Despite it being important in modern society, few people think about how this, and other chemicals, are manufactured.
Methanol can be produced by reacting natural gas with steam and oxygen at high pressures and temperatures to produce synthesis gas, which is a mixture of carbon monoxide, carbon dioxide and hydrogen. When this gas is passed through a chemical reactor under the right conditions, and in the presence of a catalyst, methanol is formed. Chemical engineers are responsible for the design, construction and, safe and efficient operation of the processing facilities that produce the methanol.
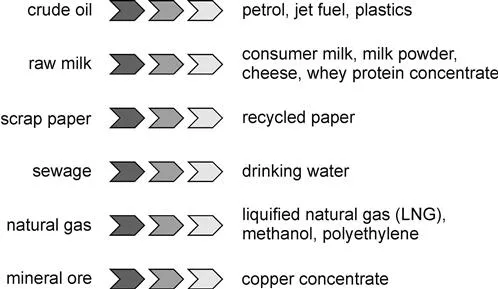
If there is engineering to be done on a process, then it is a chemical engineer who will do that. Chemical engineers are those people who are behind the development and operation of many of today's most important processing facilities (Figure 1.1). They are the people whose work ensures that every day, there is water that is safe to drink, fuel to power cars, trucks and planes, pharmaceuticals to treat a range of medical conditions, metals, plastics and a range of synthetic materials to meet the demands of the modern consumer, and food that is safe to eat. A conventional image of the chemical engineer is as a professional who works in the oil and gas, or petrochemical industries designing, building and operating large facilities such as refineries. Today, however, chemical engineers work in many more industries ranging from minerals processing and the energy sector to the pharmaceutical and the food and beverage industries.
Figure 1.1 If a process must be engineered, then a chemical engineer will be involved.
Chemical engineering is the profession in which knowledge of mathematical, chemical, biological and physical sciences, gained by study, experience and practice is applied, with judgement, to develop processes that take one or more raw materials and safely and economically transform that material into, either more useful, or less harmful, products.
Chemical engineers are the engineers who design, build and operate oil refineries that take crude oil and by using chemical and physical processes transform the oil into a range of products from petrol of a variety of grades, to jet fuel, bunker oil, lubricants and plastics. The operation of the refineries is flexible, allowing them to process the oils from different oil fields with their widely different properties.
While the oil and gas, and petrochemical industries are huge, so too are the food and beverage industries, which also employ chemical engineers. Chemical engineers design the processes that take raw milk and transforms that into a range of consumer milk products from skim milk to cream, all of which must be safe to consume. The production of cheese makes use of a biological process in which bacteria is added to the milk, fermenting the milk's lactose into lactic acid.
Chemical engineers also are in the forefront in processing waste material so that it might be reused in some form. For example, they are involved in the design, construction and operation of paper mills in which used paper is recycled into new, paper-based products.
To begin our study of chemical engineering we will consider two processes in more detail:
- the production of consumer milk from raw milk;
- the production of methanol from natural gas, steam and oxygen.
1.1 The Production of Consumer Milk
Raw milk, produced on a farm, is unsafe to drink as it can contain micro-organisms that, if left untreated, might cause illness to anyone drinking it. The solution to make the milk safe to drink is to heat the milk to around 75 °C and then to hold it at that temperature for around 15 s, before rapidly cooling it to around 1 °C to 4 °C. The heat kills enough of the pathogens to allow the milk to be consumed, without risk of illness. The process, known as pasteurisation, was developed by the French scientist Louis Pasteur during the nineteenth century.
In pasteurising the milk, it is important that it is heated to 75 °C ±1 °C, and that it is held within that temperature range for 15 s. If the milk is not heated to a high enough temperature, then not all the micro-organisms will be killed. If 75 °C is exceeded, then the pathogens will be killed, but the milk will deteriorate and the taste will be unacceptable to the consumer. If the milk is heated to the correct temperature, but is then held there for less than 15 s, then not all the micro-organisms will die. Holding the milk at 75 °C for longer than 15 s will kill all the pathogens, but again, the taste of the milk will be unacceptable. The process needs to be controlled such that the correct temperature is achieved for the right period of time.
As well as pasteurising the milk, it is also common to both standardise and homogenise the milk. In many countries, consumers demand a range of milk products with lower fat contents than raw milk. Where raw milk might contain around 4% fat, there is a demand in many markets for milk with either 1%, 2%, 3% or 4% fat content. Standardisation is the process in which the fat content of milk is kept at a fixed value, even when the fat content of the milk arriving at the dairy processing facility varies. The standardisation process involves separating the raw milk into two streams – one that is essentially cream, rich in fat, and another that is skim milk, with almost no fat content. The two streams are then blended to produce the milk with the desired fat content.
A layer of cream will form on the top of a container of milk, if that milk has been left to stand for some time, and if the milk has not been homogenised. In the homogenisation process, the fat globules present in the milk are broken down in size and distributed throughout the milk. This prevents the fat globules from coming together to form the cream layer on the top of the milk.
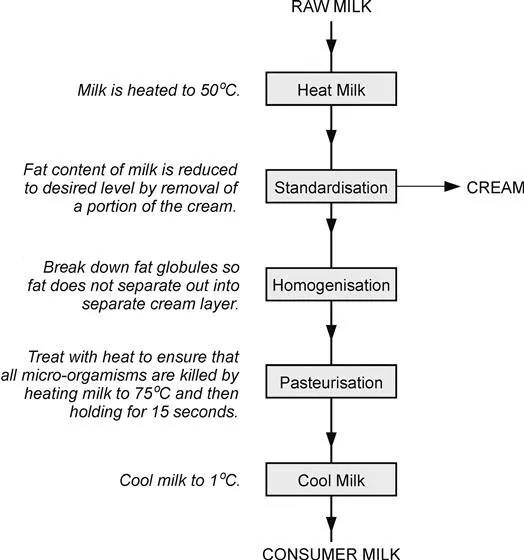
The basic steps in the process to produce consumer milk from raw milk are shown in Figure 1.2. The raw milk is first heated to 50 °C and is then standardised. The unwanted fat is removed from the milk in a stream of cream. The fat globules are next broken down in size in the homogenisation processes to prevent the remaining fat from forming a cream layer at the top of the milk. In the next stage, the milk is heated to 75 °C and held at that temperature for 15 s before being cooled to 1 °C.
Figure 1.2 The basic steps in producing milk that has been standardised, homogenised and pasteurised.
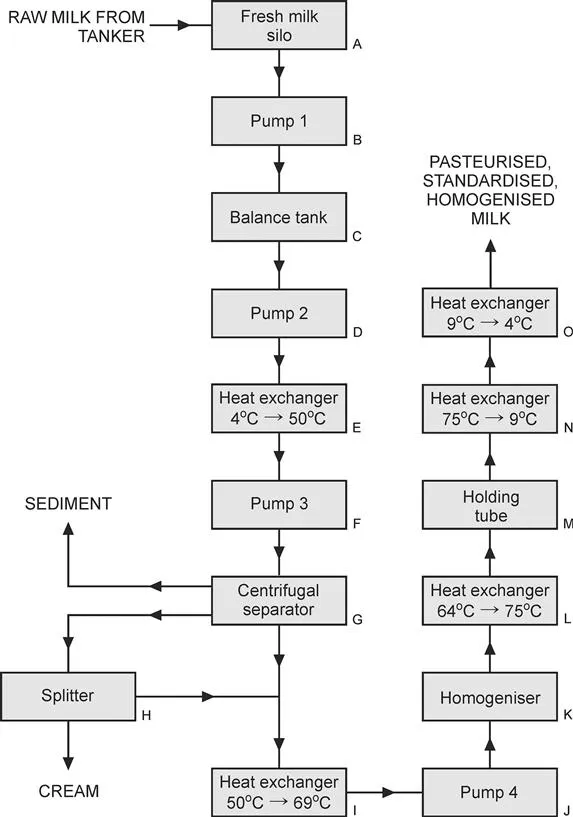
Now let us go into a little more detail about the operation of a dairy facility that processes milk through standardisation, homogenisation and pasteurisation. Let us consider the more detailed process as shown in Figure 1.3.
Figure 1.3 More detailed steps for producing milk for the consumer.
Milk arrives at the dairy processing facility by refrigerated road tanker after having been picked up from a range of dairy farms in the district. The fat content of the milk delivered to the facility might vary with a number of factors including the weather, location and feed available to the dairy herd. On arrival, the milk is pumped into the fresh milk refrigerated silos (A in Figure 1.3). For this example, we will assume that the average fat content of the milk in the silo is 4.2% (i.e., in a 100-kg sample of the milk, 4.2 kg would be fat, and 95.8 kg would be nonfat content such as skim milk, proteins and other solids). The fresh raw milk is pumped via a centrifugal pump (B) from the fresh milk silo to the balance tank (C) at an average rate of around 20 000 l h−1. The role of the balance tank is to maintain the inlet pressure into pump 2 (D) at a constant value.
Milk is pumped from the balance tank using pump 2, inlet to the first heat exchanger. This plate heat exchanger raises the temperature of the milk to around 50 °C (E). From here pump 3 (F) sends the milk to the centrifugal separator (G). This specialist item of equipment is unique to the dairy industry. A stack of concentric bowl-shaped sieves rotating at 3500 revolutions per minute (rpm) act to separate the incoming milk into two streams, one that is rich in fat (the cream stream), and one which has a very low fat content (skim milk). Approximately 2350 l h−1 of cream with a fat content of 37% flows to a splitter (H), while around 17 600 l h−1 of skim milk with a fat content in the range of 0.05 to 0.1% leaves the separator in the other stream. The discharge pressures of the cream and skim milk lines are 500 kPa and 420 kPa, respectively. Sediment including straw, dirt and dead cells are also collected in the separator and are automatically discharged every 20 min by the separator.
The splitter (H) is used to regulate the final fat content of the produced milk. If a higher fat content is desired in the product milk, then a greater proportion of the cream is reintroduced from the splitter back into the production line. If a lower fat content is desired, then more of the cream product is removed from the process.
The pressure of the blended milk is sufficient to allow the milk to flow through the next heat exchanger (I) that takes the milk up to 69 °C. Pump 4 (J) then drives the milk through the homogeniser. In this unit, the milk is forced through small holes that act to break up the fat globules into very much smaller globules. These smaller globules are dispersed throughout the milk and will not come together again to form cream layers. The discharge pressure from the homogeniser is sufficient to force the milk through the remaining heat exchangers.
The milk that has been standardised and homogenised is now heated to 75 °C by indirect heat exchange with hot water. The hot milk then passes through a holding tube (M), the length of which has been calculated so that it takes 15 s for the milk to pass through the tube. On exiting the tube, the milk is then passed through a plate heat exchanger (N) that lowers the temperature rapidly to 9 °C. In the final stage of the process the milk's temperature is lowered to 4 °C by indirect heat exchange with chilled water at 1 °C. The chilled milk is then pumped to storage silos, from where it is pumped to the packing lines as required.