GILES M. PRENTICE, LIAM EMMETT, VIJAY LUXAMI AND G. DAN PANTOŞ*
1.1 Introduction
1,4,5,8-Naphthalenediimides (NDI) represent a class of aromatic compounds with an electron-deficient π-system whose electronic, redox and spectroscopic properties have led to their extensive study in the field of supramolecular and materials chemistry. Owing to their planar aromatic nature, naphthalenediimides can exhibit aromatic π-stacking and van der Waals interactions. The electron density on most aromatic rings create a quadrupole moment with a partial negative charge above and below the face and a partial positive charge around the periphery. When electron-deficient NDI is paired with complementary electron-rich species, it adopts an optimum face-centered stacking often referred to as a “donor–acceptor” complex. Different spectroscopic studies have shown that association of π-donor and π-acceptor molecules is more favourable than the self-association of π-donor–donor and π-acceptor–acceptor scaffolds.1,2 The π system of the NDI core is amenable to anion–π interactions and has been exploited extensively in the construction of artificial anion channels.3,4 The carbonyl oxygen atoms have been shown to interact with metal cations and form a multitude of hydrogen bonding arrays, while the electron withdrawing nature of the imide groups installs a significant partial positive charge on the aromatic C–Hs, which allows them to participate in non-classical C–H⋯O hydrogen bonds.5 The chemistry of NDIs has developed rapidly in the past 15–20 years due to this multitude of supramolecular handles present on the NDI core and their straightforward synthesis.6–9 A number of reviews have covered parts of this field10,11 and this chapter will highlight a few of the main areas in which NDIs and their congeners have driven supramolecular chemistry forward.
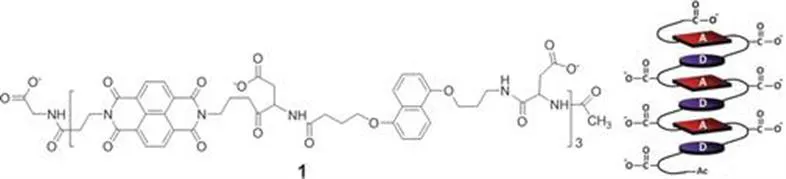
Iverson et al. reported aedamers (aromatic electron donor–acceptor) as the first foldamers to make use of aromatic π–π interactions in water to direct folding. The aedamers exploited the π-acidity of NDI to form the presumed pleated structures based on the complementary face-to-face association of alternating an electron-rich “donor”, 1,5-dialkoxynaphthalene (DN) and an electron-deficient “acceptor”, NDI.1,12,13 Aspartic acid residues were linked in between aromatic moieties to facilitate solubility in water (Figure 1.1). These types of NDI-intercalators form strong complexes with DNA, which dissociate extremely slowly, corresponding to a half-life of 16 days.14
Figure 1.1 An NDI–DN aedamer structure and the pleated cartoon representation.12
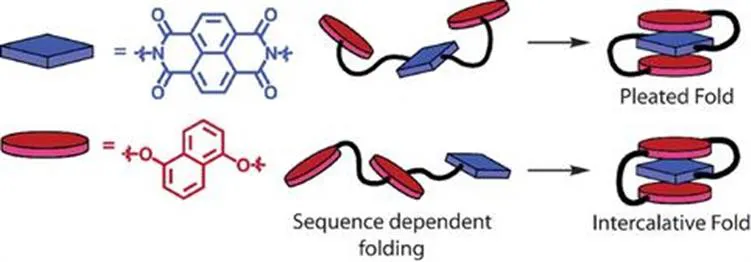
The effect on folding of the D–A interaction was investigated further by Iverson et al. in a study where water-soluble trimers were synthesised with different sequences of NDI and 1,5-DN (Figure 1.2).13 Again, it was found that the folding always yielded a conformation in which face-centred stacking was observed between the NDI and 1,5-DN, which further reinforces the directionality of NDI–DN D–A interactions as shown in Iverson and co-workers’ previous work.
Figure 1.2 Folding in NDI–DN trimers.13
1.2 Molecules with Complex Topologies
NDI and BDI have been used extensively in catenanes and rotaxanes. Their properties as acceptors and relative ease of electrochemical reduction have led to a number of templated syntheses of catenanes, as well as properties such as bistability and even potential molecular machines.
Sanders and co-workers in 1998 synthesised a variety of NDI, BDI and DN [2]-catenanes by use of D–A interactions.15 Crown ethers containing 1,5-DNs were synthesised, where terminally alkyne appended NDIs or BDIs were then coupled using CuCl2 in the presence of these ethers (Figure 1.3).
Figure 1.3 A donor–acceptor [2]-catenane synthesised by Sanders et al.15
The formation of [2]-catenanes was obtained as a diimide bound between the DNs in the macrocycle affording the interlocked structure in yields between 29 and 52% for BDI and NDI respectively. The stronger binding of the NDI to the DN when compared to a BDI–DN interaction was confirmed by a competition experiment. A series of modifications on this catenane were later developed by the Sanders group, which showed the versatility of the building blocks used in this approach.
Firstly, a hybrid butadiyne crown ether macrocycle was synthesised and catenation by the original method could be prevented by the use of Co2(CO)6 to deform the macrocycle by binding to alkynes. This process was found to be reversible by removal of the Co2(CO)6. It was also possible to assemble the catenane in a single step with CuCl2-catalysed coupling of the alkyne-appended DN and BDI previously used (Figure 1.4).16–18
Figure 1.4 Synthesis through an initial step of self-assembly.18
Post synthetic modification of the butadiyne linkers was also performed by hydrogenation by Pd/C (5%). The crown ether was also altered with dialkoxydibenzoates and dialkoxybenzoates in the place of one of the DN moieties; this still allowed for the synthesis of the [2]-catenanes as previously described.17 Following these modifications, Sanders and co-workers proposed a supramolecular protection of dialkoxybenzoates using Sn porphyrins, which can be used to assemble larger supramolecular assemblies.19
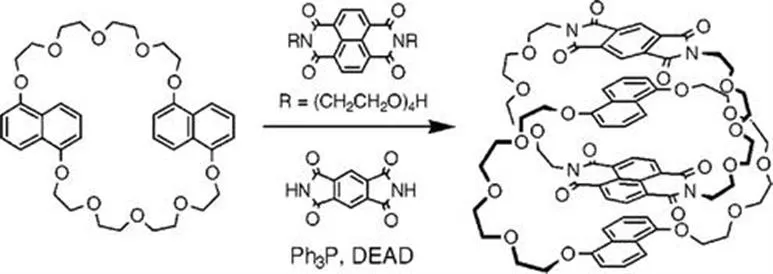
Sanders and co-workers then further developed this work synthesising [2]-catenanes with the same aromatic moieties. However, in this case, they utilised a Mitsunobu alkylation between a tetraethylene glycol appended NDI and BDI (Figure 1.5).20 As in the previous example, the DN crown ether acts as a template with the NDI bound in between the DN moieties (Figure 1.5).
Figure 1.5 An NDI–BDI–DN [2]-catenane prepared by Sanders et al.20
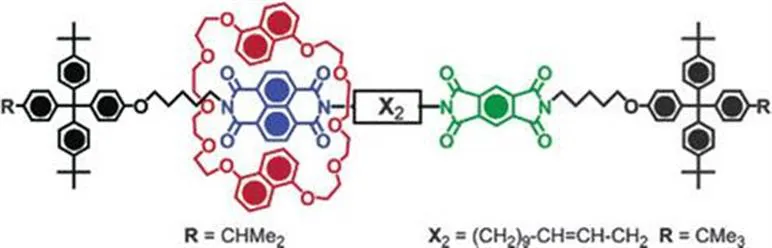
Crown ether containing DNs with NDI and BDI were again used in the synthesis of controllable [2]-rotaxanes (Figure 1.6).21,22 In this system, a number of electron-deficient stations are installed along the axle of the rotaxane on which the DN macrocycle binds. Modulating the strength of the interaction at each site can control the position of the ring on the axle. This was achieved by two different acceptors, NDI and BDI, installed in the axle; all-NDI and all-BDI [2]-rotaxanes were also synthesised.
Figure 1.6 A bistable [2]-rotaxane by Sanders and Stoddart et al.21
While the NDI station is the preferred binding site due to its larger π surface, its properties can be modulated electrochemically or chemically, leading to a switching of the rotaxane conformation. The redox-induced switching was performed by cyclic voltammetry in CH2Cl2. Firstly, the NDI site is deactivated by electrochemical reduction (−0.74 V) and the BDI site becomes the preferable acceptor, however, further electrochemical reduction (−1.06 V) can deactivate this site leading to no donor–acceptor interactions. Interestingly, with the all-NDI and BDI, each site was found to have a different reduction potential.
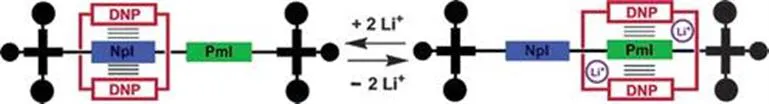
Chemical switching could be obtained by addition of lithium cations as in the presence of lithium ions, the DN crown ether sits on the BDI as the interaction between the ether loop and the cations is stronger than in the NDI (Figure 1.7). This process was observed by 1H NMR and UV-vis spectroscopy.21
Figure 1.7 Cation-triggered switching in a bistable [2]-rotaxane. NpI=NDI, PmI=BDI, DNP=DN.21
This work was further extended in 2008 when Stoddart and co-workers synthesised a [2]-catenane composed of the same DN crown ether with two acceptor sites: an NDI and 1,4-bipyridinium separated by two tetraarylmethane un...