![]()
Section II
![]()
2 Polyketide Natural Products
2.1 Introduction
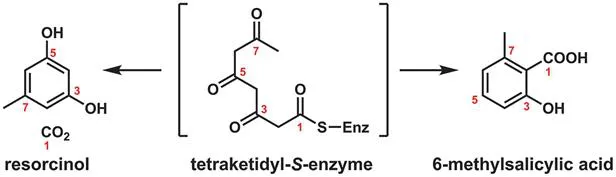
The first major class of natural products to be analyzed is the diverse set of molecules that comprise the polyketides (Dewick 2009, Mander and Liu 2010). The first molecule synthesized and structurally characterized by Collie in 1893 was the simple 5-methyl-1,3-catechol resorcinol (Staunton and Weissman 2001). The hypothesis that polyketides originated from head to tail condensation of acetate-derived units was worked out for 6-methylsalicylate by Birch in 1955 using radioactive tracer studies (Figure 2.1). Nuclear magnetic resonance (NMR) analyses of feeding studies with doubly 13C-labeled acetates, trideuteromethyl acetate, and 18O-labeled acetate isotopomers subsequently allowed detailed hypotheses about biosynthetic pathways for many scaffold variants in this natural product class.
Figure 2.1 Resorcinol and 6-methylsalicylic acid: two phenolic polyketides of historical significance.
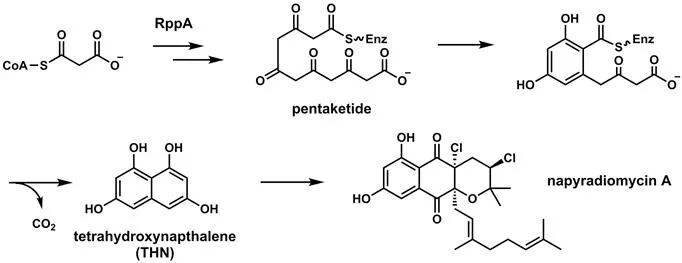
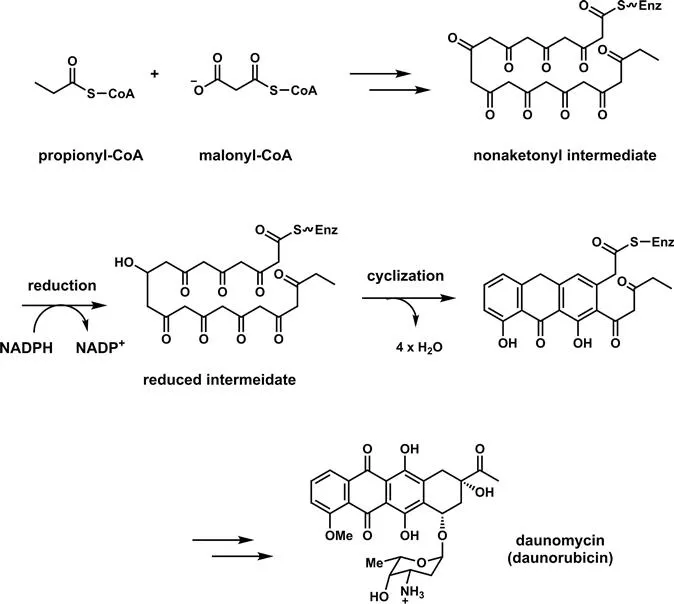
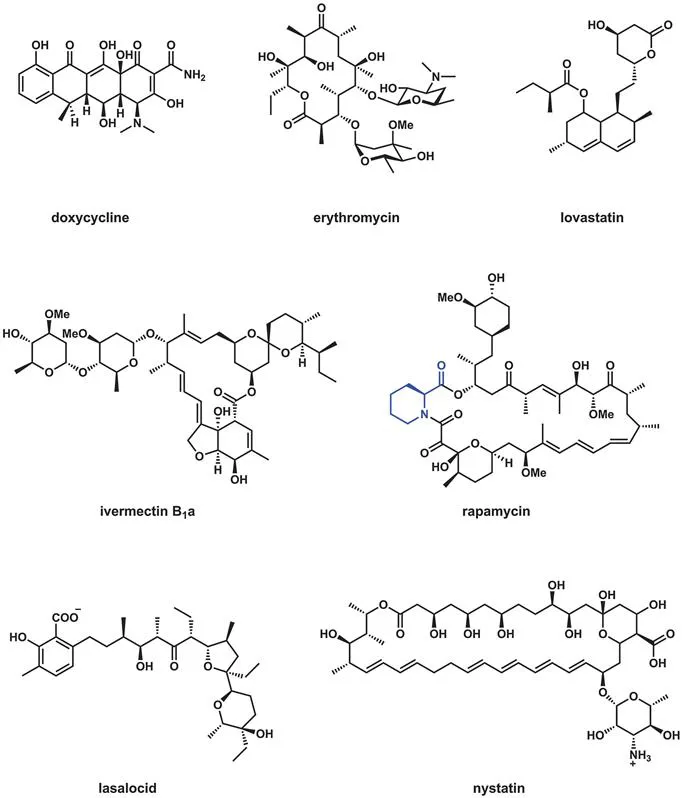
Among several possible definitions of polyketides as complex natural products is a biosynthetic one, that the final products arise from polyketonic intermediates. This is valid for aromatic polyketides, including the bicyclic tetrahydroxynapthalene core in napyradiomycin A (Figure 2.2) which features a tetraketonyl thioester intermediate. It also holds for biosynthesis of the antitumor agent daunomycin (Figure 2.3) where a linear nonaketonyl thioester enzyme intermediate undergoes three aldol cyclizations and one thioclaisen condensation to release the characteristic fused aromatic tetracyclic framework. Unfortunately this is not true for other structural types of polyketides, among them erythromycin, ivermectin, lovastatin, rapamycin, lasalocid, and nystatin, shown in Figure 2.4. Monoketonic but not polyketonic intermediates are generated during assembly.
Figure 2.2 The tetrahydroxynaphthalene core of napyradiomycin A arises from a tetraketonyl thioester enzyme intermediate.
Figure 2.3 The fused tetracyclic aromatic scaffold of the anticancer agent daunomycin arises by intramolecular aldol condensations from a covalent decaketidyl-S-enzyme, with nine keto groups.
Figure 2.4 Seven diverse polyketide scaffolds of therapeutic utility.
Each of the molecules in Figure 2.4 except doxycycline is biosynthesized by iterative condensations involving β-ketoacyl thioester enzyme intermediates, like the aromatic polyketides. However, the initial β-keto group can undergo up to three kinds of successive enzymatic transformations, reduction to the β-OH-acyl thioester, dehydration of the alcohol to the α,β-enoyl thioester, and then saturation to the β-CH2 group, before the next iterative chain extension. Polyketonic intermediates are thus avoided. We shall examine these two extremes of catalytic processing in subsequent sections of this chapter. Clearly nystatin and erythromycin (Figure 2.4) have undergone some but not all three tailoring modifications of the β-keto groups, as various hydroxyl and olefinic functionalities persist in the mature scaffolds.
2.2 Polyketides Have Diverse Scaffolds and Therapeutic Utilities
Figure 2.5 notes several distinct molecular subclasses of polyketides. The macrolactone class is represented by the antibiotic erythromycin as well as the anthelminthic ivermectin (and the insecticide spinosyn; see Figure 2.29). Nystatin, with its six double bonds, is an example of the polyene antifungal subgroup, while doxycycline represents the tetracyclic aromatic class of antibiotics. The decalin-containing lovastatin, inspiration for the best selling cholesterol lowering drugs, is formed by a [4+2] cyclization, likely via a biological Diels–Alder reaction (as is spinosyn). The veterinary drug lasalocid contains a furan and a pyran ring that are the prototypic ring structures that dominate the marine toxin polyether subclass of polyketides.
Figure 2.5 Subclasses of polyketides.
Rapamycin is a widely used immunosuppressive drug that is a macrolactone but also contains the amino acid building block pipecolate inserted into the polyketide backbone. This is an example of a hybrid polyketide–nonribosomal peptide structure, a subclass examined in the next chapter after the logic of nonribosomal peptide synthetase assembly lines has been detailed. The final subclass noted in Figure 2.5 also represents an intersection of two different types of natural product biosynthetic pathways, isoprenyl groups (Chapter 4) and polyketide scaffolds. The biosynthesis of hyperforin and fumagillin is taken up in Chapters 4 and 9, respectively, when the prenylation and oxygenative tailoring strategies are discussed.
From this brief and decidedly partial enumeration of the biological and pharmacological activities of these distinct subgroups of polyketides it is clear that these molecules span a huge range of therapeutic utility. A quick scan of the structures of Figure 2.4 also reveals them to be rich in oxygen substituents but almost totally lacking in nitrogen atoms, particularly in the polyketide core frameworks. These asymmetries reflect the use of acetyl and malonyl thioester building blocks, which lack nitrogen atoms. In the next chapter we will examine how the use, instead, of amino acid building blocks, but similar assembly line logic, builds nitrogen rich peptidic scaffolds. As a final introductory comment we note that polyketide biosynthetic pathways are the domain of carbanion chemistry for providing the nucleophiles and thioesters as electrophilic carbon partners required for C–C bond assembly as acetyl and malonyl groups are incorporated and morphed into complex molecular scaffolds.
2.3 Acetyl-CoA, Malonyl-CoA and Malonyl-S-Acyl Carrier Proteins as Building Blocks for Fatty Acids and Polyketides
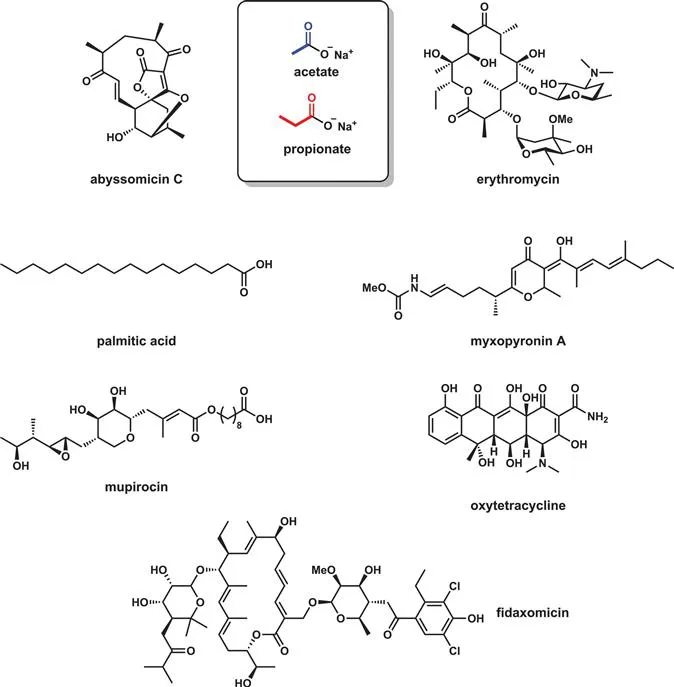
Figure 2.6 is a diagram that indicates that the two carbon acid acetate is the source of both fatty acids such as the C16 palmitic acid and the six varieties of diverse polyketide scaffolds from myxopyronin A to the topical antibiotic mupirocin. This is a dramatic oversimplification of the actual enzyme chemistry involved but makes the central point that the two carbons of acetate building blocks stay connected during iterative chain elongations of the C2 unit.
Figure 2.6 The two carbon primary metabolite acetate, activated as acetyl-CoA, is the building block for all fatty acids and polyketide classes.
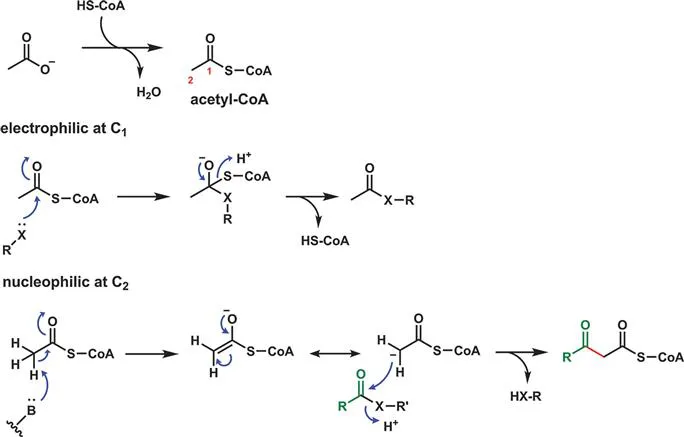
In fact, it is with the thioester acetyl-CoA that the great bulk of acetyl group metabolism occurs in both primary and secondary pathways. As the thioester, the acetyl group is electrophilic at C1 and potentially nucleophilic at C2 by virtue of stabilization of the C2 carbanion as the thioester enolate (Figure 2.7). For both fatty acid and polyketide biosynthesis, while acetyl-CoA is often the starter unit the elongation unit is instead malonyl-CoA.
Figure 2.7 Acetyl-CoA is doubly activated in the acetyl moiety: as a nucleophilic thioester enolate-stabilized carbanion at C2 and as an electrophilic carbonyl at C1 by virtue of the thioester linkage.
The malonyl thioester can undergo facile enzyme-mediated decarboxylation to lose CO2 irreversibly and generate the acetyl-CoA C2 carbanion directly. The loss of CO2 drives carbanion formation in the biosynthetic direction. This decarboxylative thioclaisen condensation is the sole reaction type for fatty acid and all subclasses of polyketide chain elongations. Figure 2.8 also shows how malonyl-CoA is generated by ATP-dependent carboxylation of acetyl-CoA by acetyl-CoA carboxylase, the first committed enzyme for both fatty acid and polyketide biosynthesis (Broussard, Price et al. 2013). While acetyl-CoA carboxylase generates malonyl-CoA, the malonyl group is transferred to a thiol on a posttranslationally modified side chain of an 8–10 kDa protein, termed an acyl carrier protein (Majerus, Alberts et al. 1965, Majerus, Alberts et al. 1965, Vagelos, Majerus et al. 1966) before utilizati...