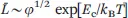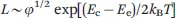![]()
CHAPTER 1
Wormlike Micelles: An Introduction
CÉCILE A. DREISSa
a King’s College London, Institute of Pharmaceutical Sciences, 150 Stamford Street, SE1 9NH, UK
Email:
[email protected] This short introductory chapter will provide the reader, and in particular those new to the field of wormlike micelles (WLMs), some basic luggage to equip them to embark onto the following chapters. The principles of WLM formation are presented, basic nomenclature is introduced, and fundamentals on the structural features of WLMs and their rheology are presented.
1.1 Why Do Wormlike Micelles Form?
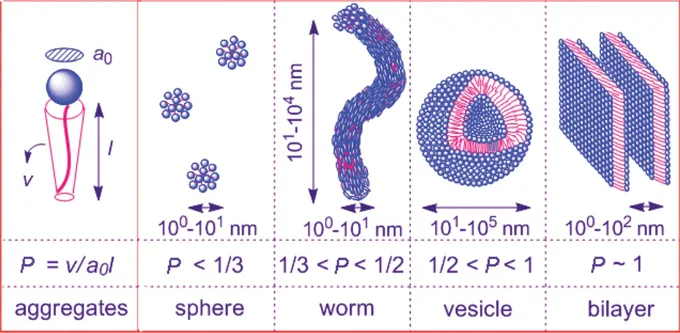
The incompatibility between the polar and apolar regions of amphiphilic molecules leads to their segregation in solvents that are selective: ‘good’ for one region, ‘poor’ for the other. In water, the polar (hydrophilic) region is referred to as the ‘head’, while the apolar (hydrophobic) section is the ‘tail’. Surfactants spontaneously self-assemble into a variety of structures, the morphology of which is dictated by the spontaneous curvature or packing parameter P of the amphiphile (Figure 1.1).1
Figure 1.1 Schematic illustration of the relationship between the packing parameter P and the morphology of self-assembled surfactant aggregates. Reproduced from ref. 2 with permission from the Royal Society of Chemistry.
The spontaneous curvature in solution merely reflects molecular asymmetry, i.e. the difference in effective packing area of the different molecular regions of the molecule (solvent-liking and solvent-hating). How molecules pack is thus dependent on the size of these antagonistic sections and their rigidity, but also the interactions present (such as hydrogen bonding or electrostatic forces), which can be tuned by parameters such as temperature, ionic strength, or pH (see Chapter 6). A high value of the spontaneous curvature reflects highly asymmetric molecules, and a tendency to assemble into spherical aggregates; on the other hand, low-curvature aggregates arise from relatively symmetric molecules and locally flat interfaces.
Israelachvili introduced the concept of the ‘critical packing parameter’ P,1 a geometrical quantity defined as v/lca0, where v is the volume of the lipophilic chain having maximum effective length lc, and a0 is the effective area per molecule at the surfactant/water interface. Cylindrical aggregates (thus WLMs) are expected for intermediate values of P (between 1/3 and 1/2), while spherical micelles (high spontaneous curvature) form at lower values of P (≤1/3) and bilayers (low curvature) are found for P>½ (above P=1, reverse micelles are formed, which are described in Chapter 3) (Figure 1.1).
The spontaneous curvature accounts for enthalpic contributions. Entropic effects come into play through: (i) bending of the cylindrical micelles (conformational entropy) and (ii) topological defects, such as end-caps (which increase the entropy by increasing the number of micelles) and branching points or junctions (which increase the configurational entropy). Both end-caps and branches have in common the formation of regions with different curvatures compared to the main cylindrical body, and incur different energetic penalties.3–5 The occurrence of intermicellar junctions has been invoked in several studies to explain a drop in the zero-shear viscosity, η0, as a function of surfactant, co-surfactant, or salt concentration, since the work of Cates6 and Lequeux,7 as the junctions are free to slide along the micellar body and thus provide an additional mechanism of relaxation. The presence of branches was later confirmed by cryo-TEM imaging (Chapter 7), which is about the only technique capable of identifying their presence. Recently however, pulsed field gradient (PFG) NMR measurements have been proposed as a new technique allowing a reliable determination of intermicellar junctions (more details on this technique, and its application specifically to reverse WLMs, can be found in Chapter 3).
1.2 Which Surfactants Form Wormlike Micelles?
The best-known and most studied WLM systems are cationic surfactants with long aliphatic chains, such as cetyltrimethylammonium bromide (CTAB) or cetylpyridinium bromide (CPBr), for which micellar growth takes place at relatively high concentration or in the presence of salt (a comprehensive list of systems, and the effect of the structure of counterions, are discussed in Chapter 11). Following these initial studies on cationic surfactants, numerous surfactants have been found to aggregate into WLMs, either in the presence of smaller headgroup-co-surfactants, other additives, salts, or with appropriate counterions.8,9 Chapter 4 offers a catalogue of more unorthodox amphiphiles which have also been reported to form WLMs and differ from the ‘classic’ hydrocarbon-based surfactants.
1.3 Key Structural Parameters
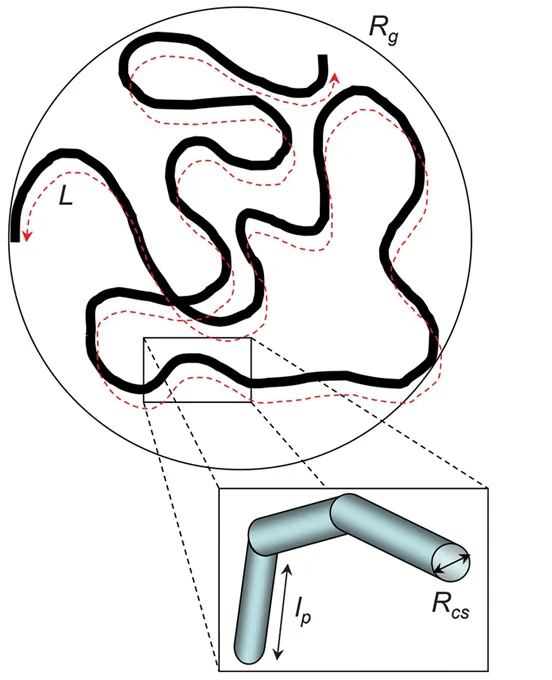
WLMs can be fully described by a number of structural parameters, which cover a broad range of length-scales. Figure 1.2 presents a schematic view of a WLM with the main dimensions of interest.
Figure 1.2 Schematic representation of a WLM showing characteristic length-scales: the overall radius of gyration Rg, contour length L, persistence length lp, and cross-section RCS. Reproduced from ref. 8 with permission from the Royal Society of Chemistry.
The overall length of the micelles is referred to as the contour length L and varies from a few nanometres to micrometres. Cryo-TEM provides a direct visualization of the micelles and can be used to estimate the contour length; direct imaging techniques for WLMs are discussed in Chapter 7. Light scattering and small-angle neutron and X-ray scattering (SANS/SAXS) have also been used extensively to examine the structure of WLMs and are reviewed elsewhere.10,11 SAXS and SANS also offer a technique of choice to unravel the kinetics and formation pathways,12 which are discussed in Chapter 11.
A mean-field treatment of the growth process for either neutral or highly screened micelles gives a prediction of the average contour length in terms of volume fraction φ, temperature, and the end-cap energy Ec required to form two hemispherical end-caps as a result of rod scission:13
For charged micelles in the absence of electrolyte, the scission energy has an additional component, Ee, due to the repulsion of charges along the backbone that favour shorter micelles. In this case the contour length L is given by:
Another important structural parameter in describing WLMs is the persistence length lp, the length over which micelles are considered rigid, which provides a measure of flexibility (it is related to the Kuhn length b by b=2lp). It is usually in the range 100 to ∼400 Å for uncharged surfactant WLMs,14 thus much larger than for polymers, due to the thickness of the cross-section (see Chapter 2 for a comparison of the rheology and structural differences between WLM and polymer solutions). For charged micelles, this value is highly dependent on the ionic strength.15–17
Unde...