
Chemical Processes for a Sustainable Future
- 800 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Chemical Processes for a Sustainable Future
About this book
This comprehensive book approaches sustainability from two directions, the reduction of pollution and the maintaining of existing resources, both of which are addressed in a thorough examination of the main chemical processes and their impact. Divided into five sections, each introduced by a leading expert in the field, the book takes the reader through the various types of chemical processes, demonstrating how we must find ways to lower the environmental cost (of both pollution and contributions to climate change) of producing chemicals. Each section consists of several chapters, presenting the latest facts and opinion on the methodologies being adopted by the chemical industry to provide a more sustainable future. A follow-up to Materials for a Sustainable Future (Royal Society of Chemistry 2012), this book will appeal to the same broad readership - industrialists and investors; policy makers in local and central governments; students, teachers, scientists and engineers working in the field; and finally editors, journalists and the general public who need information on the increasingly popular concepts of sustainable living.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
General Concepts in Sustainable Chemical Processes
*Email: [email protected]; [email protected];
[email protected]
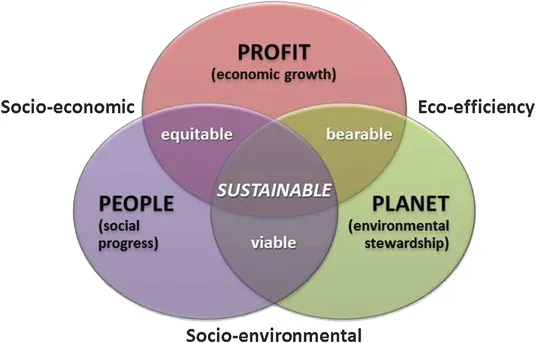
1.1 WHAT IS A SUSTAINABLE CHEMICAL PROCESS?
- People (social bottom line): worker happiness, industrial safety, benefits based on payroll expense, promotion rate, ‘loss time accident frequency’, ‘expenditure on illness and accident prevention/payroll expense’, ‘number of complaints per unit value added’, etc.
- Planet (environmental bottom line): life cycle assessment (see Section 1.4); environmental impacts such as acidification, global warming, human health, ozone depletion, photochemical ozone, wastes – hazardous and non-hazardous, and ecological health; and resource usage such as energy use, material use, water use and land use.
- Profit (economic bottom line): capital and operating costs, wealth created, value added per unit value of sales, value added per direct employee, and R&D expenditure as a percentage of sales.
1.2 THE PRINCIPLES OF GREEN CHEMISTRY AND GREEN ENGINEERING
1.2.1 The Twelve Principles of Green Chemistry
- Prevention: It is better to prevent waste than to treat or clean up waste after it has been created.
- Atom Economy: Synthetic methods should be designed to maximize the incorporation of all materials used in the process into the final product.
- Less Hazardous Chemical Syntheses: Wherever practicable, synthetic methods should be designed to use and generate substances that possess little or no toxicity to human health and the environment.
- Designing Safer Chemicals: Chemical products should be designed to affect their desired function while minimizing their toxicity.
- Safer Solvents and Auxiliaries: The use of auxiliary substances (e.g., solvents, separation agents, etc.) should be made unnecessary wherever possible and innocuous when used.
- Design for Energy Efficiency: Energy requirements of chemical processes should be recognized for their environmental and economic impacts and should be minimized. If possible, synthetic methods should be conducted at ambient temperature and pressure.
- Use of Renewable Feedstocks: A raw material or feedstock should be renewable rather than depleting whenever technically and economically practicable.
- Reduce Derivatives: Unnecessary derivatization (use of blocking groups, protection/deprotection, temporary modification of physical/chemical processes) should be minimized or avoided if possible, because such steps require additional reagents and can generate waste.
- Catalysis: Catalytic reagents (as selective as possible) are superior to stoichiometric reagents.
- Design for Degradation: Chemical products should be designed so that at the end of their function they break down into innocuous degradation products and do not persist in the environment.
- Real-time Analysis for Pollution Prevention: Analytical methodologies need to be further developed to allow for real-time, in-process monitoring and control prior to the formation of hazardous substances.
- Inherently Safer Chemistry for Accident Prevention – Substances and the form of a substance used in a chemical process should be chosen to minimize the potential for chemical accidents, including releases, explosions, and fires.
1.2.2 The Twelve Principles of Green Engineering
Table of contents
- Cover
- Title
- Copyright
- Contents
- About the Editors
- Introduction
- Chapter 1 General Concepts in Sustainable Chemical Processes
- Part A: Chemical Transformations
- Processes to Facilitate Chemical Transformations
- Examples of Chemical Transformations and Processes
- Part B: Biochemical Transformations and Reactors
- Extractions and Preparations
- Part C: Separations and Purifications
- Part D: Process Integration
- Subject Index