![]()
CHAPTER 1
Introduction
ROY M. HARRISON
School of Geography, Earth and Environmental Sciences, University of Birmingham, Birmingham, UK
1.1 WHAT IS POLLUTION SCIENCE?
There are various definitions for environmental pollution, all of which contain two key components. The first is that pollution involves some kind of change to the environment. The most obvious kinds of changes are the addition of man-made chemicals that do not occur naturally. Equally important, however, can be the additions of chemicals which do occur naturally in the environment, provided the resultant concentration meets the second criterion. This criterion is that for the phenomenon to be described as pollution, then the perturbation suffered by the environment must in some way be harmful. Not all pollution phenomena are the results of chemicals in the environment. Other important forms of pollution include thermal pollution, an example of which is the discharge of relatively warm power station cooling waters into coastal seas where they can lead to a significant change in the ecology of aquatic organisms. A further example is that of light pollution, caused by the massive amount of urban street lighting, which has a deleterious effect on the environment through obscuring our view of stars in the nighttime sky. Noise pollution has important aesthetic impacts through causing widespread annoyance, but is increasingly suspected of causing adverse effects on health.
This book concerns itself almost exclusively with chemical pollution. It considers the environment as a set of compartments that is familiar to us from our everyday existence. Therefore, separate chapters deal with the atmosphere, the world’s waters, and soils and the solid earth. While such a subdivision is convenient in that pollutants behave very differently in each of these media, it is of course not the full story. The atmosphere is very mobile and many pollutants have lifetimes in the atmosphere of only hours or days, although some remain for much longer. Pollutants in the aquatic environment, unless rapidly biodegraded, will often be present for days or weeks, and persistent pollutants for many years. In soils and sediments, however, pollutants can remain relatively immobile for tens or hundreds of years. The rates of mixing are also very different. An atmospheric pollutant with a lifetime of more than a year will become globally mixed, whereas the same degree of mixing throughout the oceans will take centuries and throughout the solid earth will never occur unless there are pathways through water and air. Exchange processes between these major environmental compartments can be very important. Thus, for example, the largest inputs of some pollutants to the North Sea arise through deposition from the atmosphere. There are other examples where pollutants discharged to the sea can become suspended in sea spray and lead to contamination of both the air and the land.
There are two main ways of monitoring chemical pollution. The first and the most obvious is through chemical analysis and that is the main focus of Chapter 5. However, there are also techniques of biological monitoring, which depend upon evaluating the effects of chemical pollution on certain sensitive organisms. Both methods have their advantages and disadvantages. Biological monitoring is rarely specific to a single substance whereas chemical monitoring is. On the other hand, chemical monitoring cannot establish an adverse effect, only a concentration, whereas biological monitoring establishes the effect rather than the concentration. The two are therefore essentially complementary. Chapter 6 examines the ecological and health effects of the chemical pollutants and Chapter 7 the institutional framework for managing the environment.
Pollution science is a relatively new discipline that brings together the various areas of traditional science, mainly from within chemistry, physics and biology, necessary to understand the behaviour of pollutants in the environment, to appreciate their effects on the environment and humans, and to monitor and manage those pollutants.
1.2 THE CHEMICALS OF INTEREST
A very wide range of chemical substances are considered in this book. They fall into three main categories:
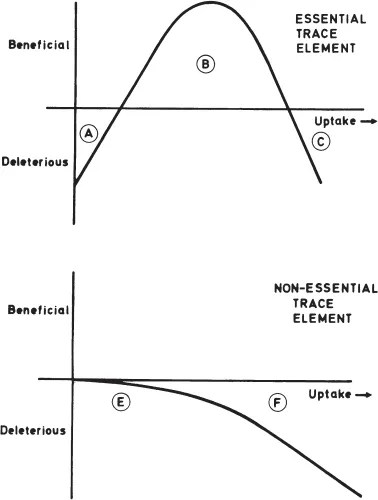
(a) Chemicals of concern because of their human toxicity. Some metals such as lead, cadmium and mercury are well known for their adverse effects on human health at high levels of exposure. These metals have no known essential role in the human body and therefore exposures can be divided into two categories (see Figure 1). For these non-essential elements, at very low exposures the metals are tolerated with little, if any, adverse effects, but at higher exposures their toxicity is exerted and health consequences are seen. In the case of the so-called essential trace elements (see Figure 1) the human body requires a certain level of the element, and if intakes are too low then deficiency syndrome diseases will result. These can have consequences as severe as those which result from excessive intakes. In between, there is an acceptable range of exposures within which the body is able to regulate an optimum level of the element. Fluoride is an example of a chemical with a very narrow window of optimal exposure. Fluoridation of water supplies is typically at a level of about 1mg L−1. Half of this concentration may still result in deficiency syndrome and weakened teeth, while double this concentration can lead to the start of adverse effects on teeth and bones.
Figure 1 Comparison of the consequences of exposure to essential and non-essential trace elements. For the essential trace elements, Region A represents the deficiency syndrome when intakes are insufficient, Area B is the optimum exposure window and in Area C, excessive intake leads to toxic consequences. In the case of the non-essential trace elements at low exposures (Zone E) the element is tolerated and little if any adverse effect occurs. In Zone F toxic symptoms are developed
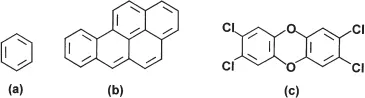
Environmental exposure to chemical carcinogens is very topical despite the minuscule risks associated with many such exposures at typical environmental concentrations. Examples of chemical carcinogens are benzene (largely from vehicle emissions), and polynuclear aromatic hydrocarbons (generated by combustion of fossil fuels). Figure 2 shows the structures of benzene, benzo(a)pyrene (the best known of the carcinogenic polycyclic aromatic hydrocarbons), and 2, 3, 7, 8-tetrachlorodibenzodioxin (the most toxic of the chlorinated dioxin group of compounds). Despite great public concern over the emissions of the last compound, the evidence for carcinogenicity in humans is quite limited.
(b) Chemicals, which cause damage to non-human biota but are not believed to harm humans at current levels of exposure. Many elements and compounds come into this category. For example, copper and zinc are essential trace elements for humans and their environmental exposures very rarely present risk to health. These elements are, however, toxic to growing plants and there are regulations limiting their addition to soil in materials such as sewage sludge which are disposed of to the land. Another category of substance for which there is ample evidence of harm to biota, but as yet little, if any hard evidence of impacts on human populations, are the endocrine-disrupting chemicals. These synthetic chemicals mimic natural hormones and can disrupt the reproduction and growth of wildlife species. Thus, for example, bis-tributyl tin oxide (TBTO) interferes with the sexual development of oysters and its use as an anti-fouling paint for inshore vessels is now banned in most parts of the world. A wide range of other chemicals including polychlorinated biphenyls (PCBs), dioxins and many chlorinated species are also believed to have oestrogenic or androgenic potential, although the level of evidence for adverse effects is variable.
(c) Chemicals not directly toxic to humans or other biota at current environmental concentrations, but capable of causing environmental damage. The prime example is the CFCs, which found widespread use precisely because of their stability and low toxicity to humans, but which at parts per trillion levels of concentration are capable of causing major disruption to the chemistry of the stratosphere.
Figure 2 Some molecules believed to have human carcinogenic potential: (a) benzene; (b) benzo(a)pyrene; and (c) 2,3,7,8-tetrachlorodibenzodioxin
1.3 UNITS OF CONCENTRATION
The concentration units used in environmental pollution are often confusing to the newcomer. Concentrations of pollutants in soils are most usually expressed in mass per unit mass, for example, milligrams of lead per kilogram of soil. Similarly, the concentrations in vegetation are also expressed in mg kg−1 or μg kg−1. In the case of vegetation and soils, it is important to distinguish between wet weight and dry weight concentrations, in other words, whether the kilogram of vegetation or soil is determined before or after drying. Since the moisture content of vegetation can easily exceed 50%, the data can be very sensitive to this correction.
In aquatic systems, concentrations can also be expressed as mass per unit mass and in the oceans some trace constituents are present at concentrations of ng kg−1 or μg kg−1. More often, however, the sample sizes are measured by volume and concentrations expressed as mg L−1 are expressed as parts per million (ppm), μg L−1 as parts per billion (ppb) and ngL−1 as parts per trillion (ppt). This is unfortunate as it l...