1.1 Molecular Amphiphiles
Molecular amphiphiles are molecules that have both a hydrophilic part and a hydrophobic part linked by covalent bonds. With their ability to significantly decrease surface tension, amphiphiles can self-assemble at air–liquid or liquid–liquid interfaces, leading to the formation of organized molecular assemblies such as Langmuir monolayers, micelles, vesicles and emulsions. Amphiphiles are therefore used extensively as detergents, emulsifiers, solubilizers and foaming agents, and are essential in a number of modern industries, from mining and manufacturing to textiles and medicine. Amphiphiles such as phospholipids are crucial components of living organisms because they are the primary constituents of biological membranes, which are essential in the structure of cells. Membranes provide a compartmentalized microenvironment, ensuring that biochemical processes occur in a systematic rather than a chaotic manner, enabling the existence of life itself.
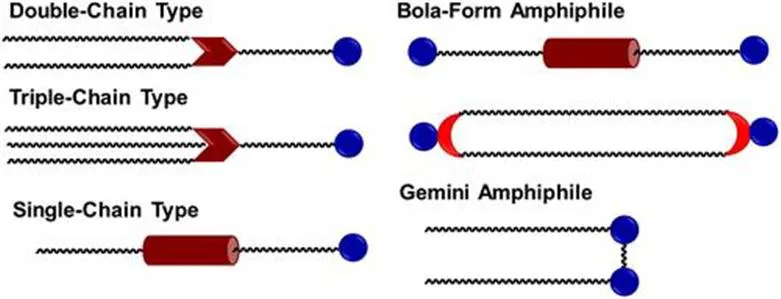
Molecular amphiphiles can be of different topologies (Figure 1.1) in terms of the different motifs acting as the linkage between the hydrophilic and hydrophobic parts.1 A hydrophilic headgroup can be covalently linked to single, double or triple hydrophobic alkyl chains. The use of synthetic molecular amphiphiles to form vesicle-like structures dates back to the 1977 paper by Kunitake et al.2 Two hydrophilic headgroups can be covalently linked by one or two hydrophobic alkyl chains, resulting in the so-called bola-form amphiphiles.3,4 Two molecular amphiphiles covalently linked at their charged headgroups are classified as gemini amphiphiles.5 The varied molecular amphiphiles have different properties, leading to different applications. For example, the critical micelle concentration of gemini amphiphiles is typically lower than that of conventional single-tailed amphiphiles, resulting in more stable micelles. The phase transition temperature of bola-form amphiphiles can be higher than that of conventional molecular amphiphiles. In other words, if a biological membrane contains a high percentage of bola-form amphiphiles, it remains stable at relatively high temperatures, which is essential for organisms that survive in harsh environments such as hot springs and submarine volcanoes.
Figure 1.1 Different structures of molecular amphiphiles obtained by molecular engineering.
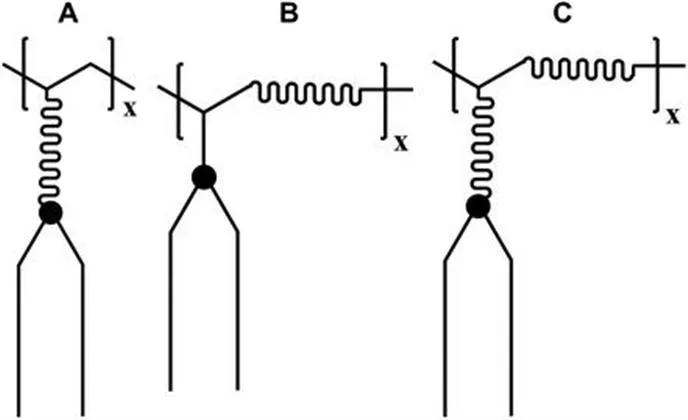
Polymeric amphiphiles can self-assemble into structures with enhanced stability and structural diversity relative to the small molecular amphiphiles. The problem with polymeric amphiphiles is the two-dimensional (2D) orientation of the three-dimensional polymer coil. Ringsdorf et al.6 introduced the concept of a flexible spacer into polymeric amphiphiles to overcome this problem. Figure 1.2 shows that there are three ways to introduce flexible spacers. Homopolymers with side-group spacers (Figure 1.2A) have been synthesized from lipids containing a hydrophilic spacer group between the membrane-forming amphiphilic part and the polymerizable unit. Hydrophilic comonomer amphiphilic copolymers with main-chain spacers (Figure 1.2B) can be prepared by the copolymerization of simple lipids. The combination of both spacer types (Figure 1.2C) can be realized by the copolymerization of spacer-containing lipids with hydrophilic comonomers.
Figure 1.2 Three possible ways to introduce hydrophilic spacer groups into polymeric amphiphiles: (A) side-group spacer; (B) main-chain spacer; and (C) main-chain and side-group spacer.
Amphiphilic block copolymers are another kind of polymeric amphiphile. As reported by Eisenberg et al.,7 amphiphilic block copolymers can self-assemble to form vesicle-like structures. By fine tuning the chain length, monomeric unit and structural topology of both the hydrophilic and hydrophobic segments of amphiphilic block copolymers, diverse organized structures can be achieved, such as spherical micelles, cylindrical micelles, bicontinuous rods, lamellae and vesicles.8
1.2 Molecular Amphiphiles for Self-Assembly
Self-assembly is a spontaneous process of forming ordered structures from a disordered initial state. Because the hydrophobic group carried by every amphiphile strongly avoids contact with water while the hydrophilic group favors contact with water, molecular amphiphiles tend to aggregate, although this does not usually result in macroscopic-scale phase separation. The unfavorable interaction between the hydrophobic group and water can be suppressed when amphiphiles self-assemble into micelles or onto low-energy surfaces, in which case the hydrophobic group aggregates in the core and the hydrophilic group is distributed on the interface with the bulk water. In this way, the net free energy of the whole system is reduced.
Amphiphiles can self-assemble to form well-defined organized structures, such as micelles and vesicles. The driving force behind the formation of such organized structures is the hydrophobic effect. The molecular basis for self-assembly is amphiphilicity. This means that controllable self-assembly can be realized by tuning the amphiphilicity of the building blocks. For example, molecular amphiphiles can form micelles by self-assembly. By relying on supramolecular chemistry to make the amphiphiles more hydrophilic, we can destroy the molecular basis for self-assembly, leading to the disassembly of micelles. When the amphiphilicity is recovered due to the noncovalent nature of supramolecular chemistry, the destroyed micelles can reform.9
1.2.1 One-Dimensional Assemblies from Molecular Amphiphiles
Micellar structures with a defined size and shape can be used in applications such as templates for making nanostructured materials and mimicking biomineralization processes. Engineering robustness is required to make these applications practical. However, micellar structures are inherently dynamic and fluid. The most straightforward method of stabilizing micellar structures is based on a covalent approach, mainly using polymerizable amphiphiles.10
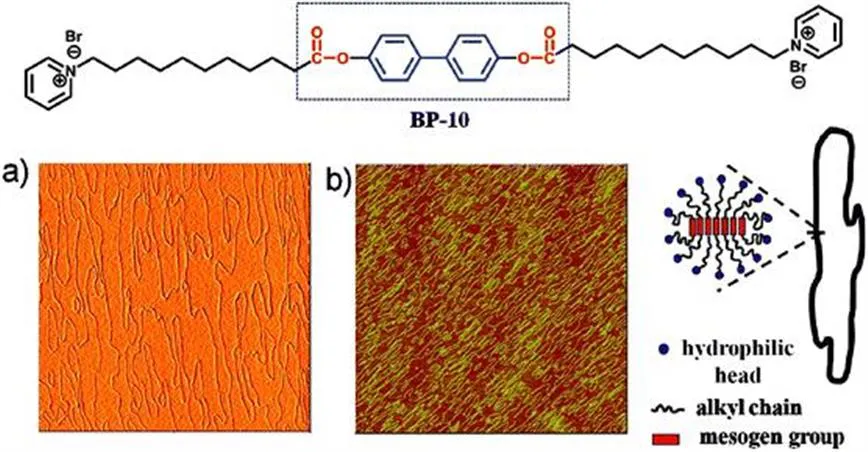
There is a supramolecular approach to stabilizing micellar structures by noncovalent interactions. To this end, we need to use an appropriate molecular design to enhance the intermolecular interactions between the amphiphiles. As an example, we designed and synthesized a bola-form amphiphile bearing a biphenyl mesogenic group and two hydrophilic pyridinium heads (BP-10).11 In situ atomic force microscopy (AFM) observations (Figure 1.3) showed that the bola-form amphiphile BP-10 formed cylindrical micelles with a spaghetti-like morphology at the mica–liquid interface. The average width of the structures was around 40 nm and some reached as long as several micrometers in length. The ex situ AFM image shows the same structure as the in situ AFM image, suggesting that BP-10 can retain the structure formed in solution. Therefore enhancing the noncovalent interactions between amphiphiles stabilizes the micellar structure against drying.
Figure 1.3 (a) In situ and (b) ex situ AFM observations of cylindrical micelles of BP-10 on a mica surface and proposed model picture. Reprinted with permission from Wang, C., Wang, Z. and Zhang, X., Acc. Chem. Res., 2012, 45, 608–618. Copyright (2012) American Chemical Society.
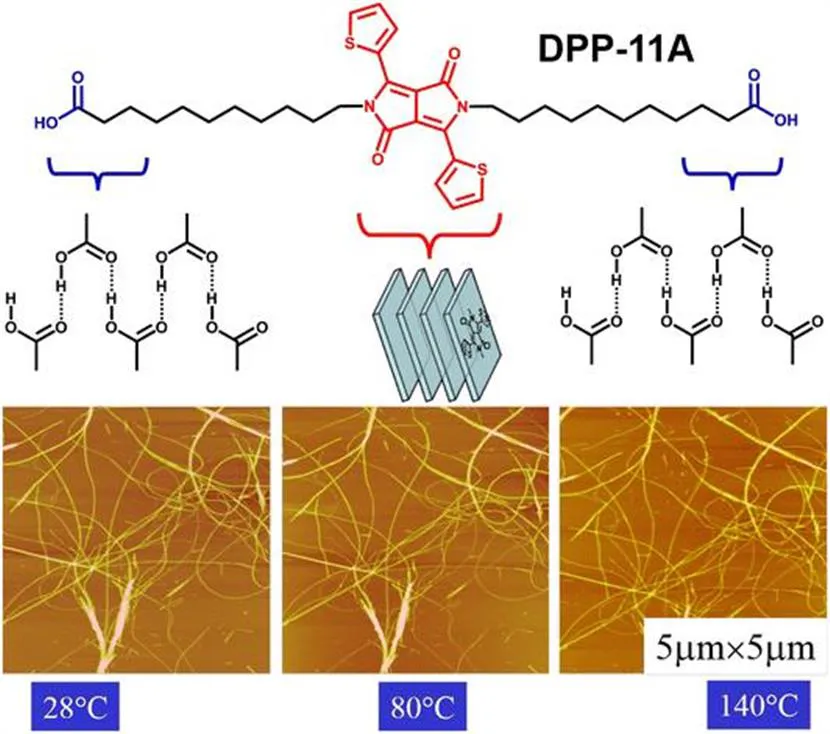
Combining various intermolecular interactions, including hydrogen bonding and π–π interactions, allows the fabrication of highly stable organized structures. For example, we designed and synthesized a bola-form amphiphile (DPP-11A) with two carboxylic acid headgroups and a diketopyrrolopyrrole (DPP) chromophore in the central part.12Figure 1.4 shows that DPP-11A can self-assemble to form supramolecular nanofibers. We tested the thermal stability of these fibers by hot-stage AFM. When the temperature was increased from 28 to 130 °C, no obvious change in the contours of the fibers was observed. When the temperature was increased to 140 °C, several parts of the nanofibers melted together. Therefore these supramolecular nanofibers have good thermostability up to 130 °C.
Figure 1.4 1D assemblies of DPP-11A showing high thermostability. Reproduced from B. Song, H. Wei, Z. Wang, X. Zhang, M. Smet and W. Dehaen, Supramolecular nanofibers by self-organiztion of bola-amphiphiles through a combination of hydrogen bonding and π–π stacking interactions, Adv. Mater., 2007, 19, 416–420. Copyright © 2007 Wiley-VCH.
1.2.2 Two-Dimensional Assemblies from Molecular Amphiphiles
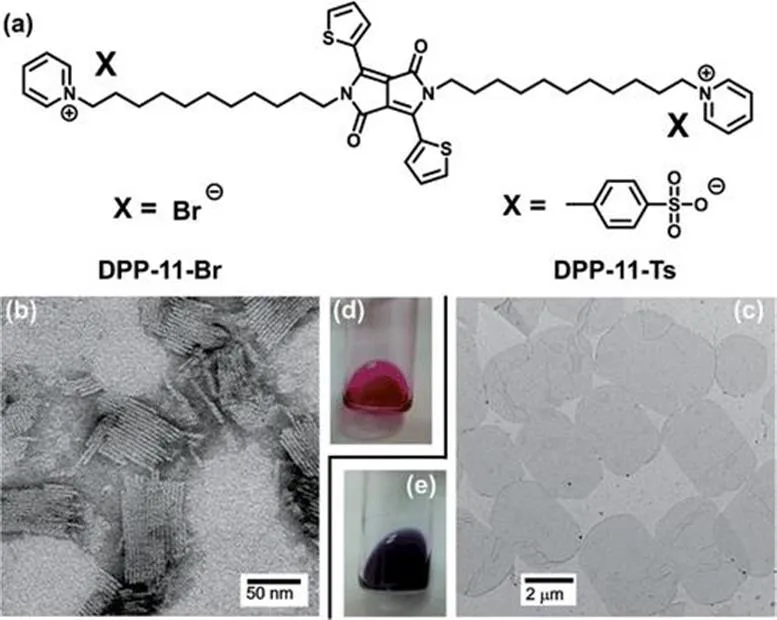
Two-dimensional planar structures possess a low curvature in addition to a smaller thickness than their planar dimensions. Bola-form amphiphiles are usually able to self-assemble to form zero- or one-dimensional (1D) assemblies. However, we demonstrated that a cationic bola-form amphiphile (DPP-11-Ts), bearing DPP as the central chromophore, can form 2D planar aggregates. Different counter anions induced different properties in these cationic bola-amphiphiles.13 To keep the same structural skeleton, the bola-amphiphiles form 1D assemblies when the counter ion is bromide, whereas they form 2D aggregates when the counter ion is tosylate. Therefore the counter ion can induce a transition from 1D to exclusively 2D planar structures (Figure 1.5).
Figure 1.5 Tuning the assemblies from a 1D structure to a 2D planar structure by varying the counter anions. (a) Molecular structures of DPP-11-X. (b) The 1D rod-like structure of DPP-11-Br, and (c...