
Oxidative Folding of Proteins
Basic Principles, Cellular Regulation and Engineering
- 429 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
About this book
The formation of disulphide bonds is probably the most influential modification of proteins. These bonds are unique among post-translational modifications of proteins as they can covalently link cysteine residues far apart in the primary sequence of a protein. This has the potential to convey stability to otherwise marginally stable structures of proteins. However, the reactivity of cysteines comes at a price: the potential to form incorrect disulphide bonds, interfere with folding, or even cause aggregation. An elaborate set of cellular machinery exists to catalyze and guide this process: facilitating bond formation, inhibiting unwanted pairings and scrutinizing the outcomes. Only in recent years has it become clear how intimately connected this cellular machinery is with protein folding helpers, organellar redox balance and cellular homeostasis as a whole.
This book comprehensively covers the basic principles of disulphide bond formation in proteins and describes the enzymes involved in the correct oxidative folding of cysteine-containing proteins. The biotechnological and pharmaceutical relevance of proteins, their variants and synthetic replicates is continuously increasing. Consequently this book is an invaluable resource for protein chemists involved in realted research and production.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
*Email: [email protected]; [email protected]; [email protected]
Disulfide bonds are unique among post-translational modifications, as they add covalent crosslinks to the polypeptide chain. Accordingly, they can exert pronounced effects on protein folding and stability. This is of particular importance for secreted or cell surface proteins, where disulfide bonds are abundant and serve to stabilize proteins against unfolding and dissociation in the extracellular milieu. However, in addition to these bonds providing security to a natively folded protein or aiding the folding process by stabilizing folding intermediates, the cysteines that form these bonds can be perilous during the maturation of nascent polypeptide chains as they enter the endoplasmic reticulum where the concentration of unfolded proteins approaches millimolar levels. This danger is even greater if the native bonds ultimately form between non-consecutive cysteines that are distant in the linear sequence or if non-native bonds are a prerequisite to achieving the final, functional structure of a protein. A wealth of exquisite detail has been obtained from in vitro studies on the biophysical effects of disulfide bonds on protein folding. Correspondingly, in-depth in vivo studies have established that the same principles apply to oxidative folding in a cell, but reveal a much more complex folding trajectory for many of the proteins that have been examined. In this chapter, we review the biophysical properties of disulfide bonds and how they affect the structure and folding of individual proteins. Based on this, we discuss similarities and differences between in vitro and in vivo folding reactions. The types of disulfide bonds that form during co-translational protein folding are described, as are the cellular strategies for accommodating this risk-laden covalent modification. We conclude with a discussion of the impact of disulfide bonds on protein misfolding and human disease.
1.1.1Stabilization of Proteins by Disulfide Bonds
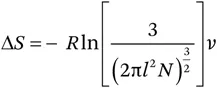
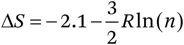
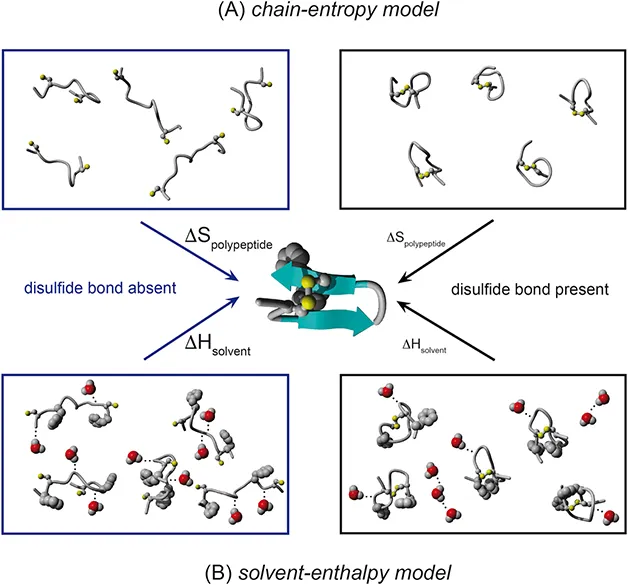
Table of contents
- Cover
- Title
- Copyright
- Contents
- Section I: Principles and Analysis of Disulfide Bond Formation
- Chapter 1.1 Disulfide Bonds in Protein Folding and Stability 3
- Chapter 1.2 Techniques to Monitor Disulfide Bond Formation and the Reduction Potential of Cysteine–Cystine Couples In vitro and In vivo34
- Chapter 1.3 Real-time Detection of Thiol Chemistry in Single Proteins
- Chapter 1.4 Analysis of Disulfide Bond Formation in Therapeutic Proteins 81
- Section II: Disulfide Bonds in Peptides and Proteins: Structure, Function and Evolution
- Chapter 2.1 Evolutionary Adaptations to Cysteine-rich Peptide Folding 101
- Chapter 2.2 In vitro Refolding of Proteins 129
- Chapter 2.3 Allosteric Disulfide Bonds 152
- Section III: Oxidative Folding in the Cell
- Chapter 3.1 Disulfide Bond Formation and Isomerization in Escherichia coli177
- Chapter 3.2 Disulfide Bond Formation in Mitochondria 205
- Chapter 3.3 Structural Insights into Disulfide Bond Formation and Protein Quality Control in the Mammalian Endoplasmic Reticulum 224
- Chapter 3.4 Mechanisms of Oxidative Protein Folding and Thiol-dependent Quality Control: Tales of Cysteines and Cystines 249
- Chapter 3.5 Disulfide Bond Formation Downstream of the Endoplasmic Reticulum 267
- Section IV: Oxidative Folding and Cellular/Organism Homeostasis
- Chapter 4.1 How Microbes Cope with Oxidative Stress 287
- Chapter 4.2 Disulfide Bond Formation in the Endoplasmic Reticulum 306
- Chapter 4.3 Redox Regulation of Hsp70 Chaperone Function in the Endoplasmic Reticulum 334
- Chapter 4.4 Thioredoxin and Cellular Redox Systems: Beyond Protein Disulfide Bond Reduction 355
- Section V: Engineering Covalent Linkages in Peptides and Proteins
- Chapter 5.1 Stabilization of Peptides and Proteins by Engineered Disulfide Bonds 381
- Chapter 5.2 Genetic Code Expansion Approaches to Introduce Artificial Covalent Bonds into Proteins In Vivo 399
- Subject Index