![]()
CHAPTER 1
Introduction: An Overview of the “What” and “Why” of Molecular Gels
RICHARD G. WEISS
Department of Chemistry and Institute for Soft Matter Synthesis and Metrology, Georgetown University, Washington, DC 20057-1227, USA
Email:
[email protected]1.1 Why Molecular Gels?
As will be discussed throughout this book, molecular gels have properties that differ from those of polymer gels, microgels, sol gels, and related forms of soft matter. One of the most important differences is that the three-dimensional (3D) networks that permeate the gels and are responsible for the immobilization of the liquid components are made up of molecules which associate through relatively weak, non-covalent, physical interactions. As a result, many of these gels can be converted reversibly and repeatedly, by heating or other simple perturbations, into their solution or sol phases; one can cycle these soft materials between viscoelastic and Newtonian fluids much more easily than one can polymer gels, in which the immobilizing networks are held together by covalent (chemical) bonds. Thus, several applications are possible for molecular gels that cannot be imagined for other forms of ‘soft matter’ (see Chapter 9).
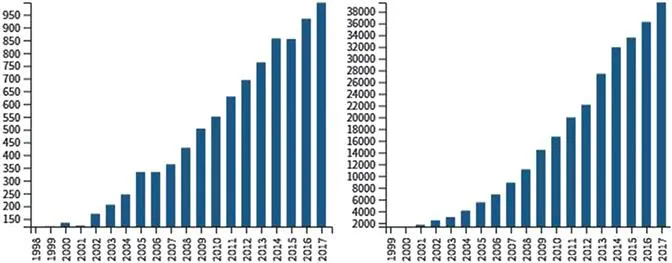
Recognition by the scientific community of these possibilities is indicated clearly by the tremendous growth in interest in molecular gels during the last three decades: from nine publications registered by the Web of Science in 1991 to 3878 in 2017; a narrower classification, using ‘molecular hydrogels’ and molecular organogels’, gives 26 articles in 1991 and 997 in 2017 (Figure 1.1). The two bar graphs show that the frequency of citations has increased even more rapidly: from 3 in 1991 to 39 544 in 2017! The interest can be attributed as well to the challenges that remain in understanding the structures and rheological properties of these gels and, especially, the dynamic processes associated with their formation. As one question concerning these materials is answered, two more arise. The study of molecular gels has been ongoing for more than a century and there is every indication that it will remain a vibrant area of research for another century.
Figure 1.1 Publications per year (left) and citations per year (right) according to the Web of Science using the combined search terms ‘molecular hydrogels’ and ‘molecular organogels’.
1.2 Before Gels–Other Self-Assembled Soft Materials
The discussion of molecular gels cannot begin without a brief introduction to the general forms of self-assembly of ‘small’ molecules. Although this book focuses on molecular gels, implying viscoelastic as well as structural attributes, it is also about a still not completely understood phenomenon: How and why do solutions and sols of some molecules aggregate and organize into objects with very large aspect ratios (i.e., 1D objects)? The leap from 1D objects to 3D networks (that are necessary for gel formation) is a fascinating process without which the applications discussed in Chapter 9 would not be possible.
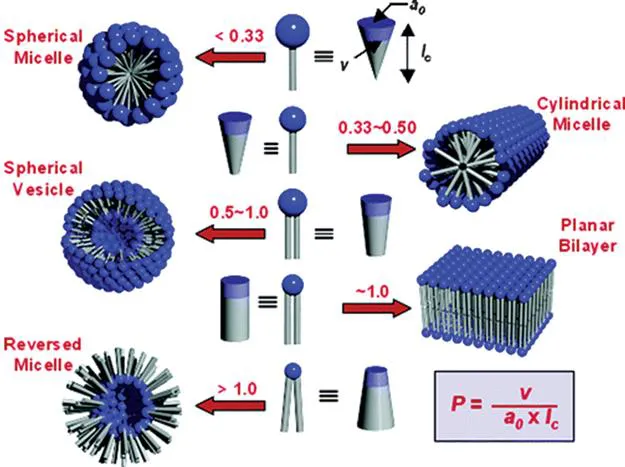
For many years, scientists have studied the self-assembly of large polymers and small molecules, and nature has shown us the importance of aggregating materials over different distance scales so that they can act synergistically and with interesting viscoelastic properties, even as living organisms.1–4 An enormous amount of effort has been expended, especially for systems in aqueous media, to understand the fundamental forces responsible for why separated and disorganized molecules (and, thus, higher in overall entropy) aggregate and organize into systems of overall lower entropy. The classic work in which Israelachvili devised an elegant (and simple) framework for our understanding of the relationships among the structures of surfactants, their concentrations, and their forms of assembly is a hallmark in this regard.5Figure 1.2 summarizes in simplistic terms the elegant relationships among these parameters for lipids with ‘melted’ chains in water: the phase packing parameter (P) can be predicted on the basis of a few structural parameters of the molecules—the head group cross-sectional area at the critical micellar concentration (a0), the alkyl chain length (lc), and the hydrophobic chain volume (v). Those concepts have been expanded beyond water by considering how the polarity and type of solvent affect the modes of aggregation.
Figure 1.2 Different self-assembled morphologies of lipids predicted from the critical packing parameter (P). Reprinted with permission from T. Shimizu, M. Masuda and H. Minamikawa, Chem. Rev., 2005, 105, 1401, Copyright 2005 American Chemical Society.
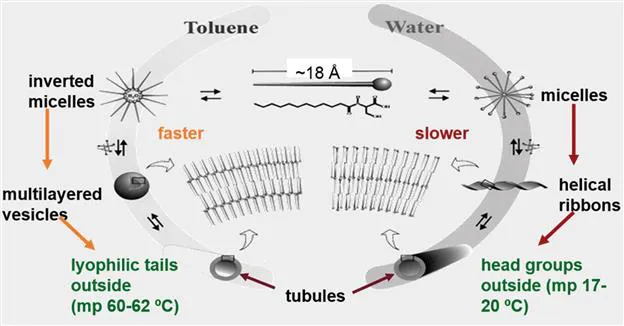
One of the most impressive examples of how incorporation of solvent can be lead to new insights is shown in Figure 1.3 where seemingly similar objects, tubules, are formed by very different routes and with very different packing arrangements depending on whether they are grown by aggregation of the lipid, L-dodecanoylserine, in water or in toluene.6 In fact, a distinction is usually made between gels in which the liquid component is aqueous (i.e., hydrogels7) and in which it is organic (i.e., organogels8), although the same principles govern both. As discussed in Chapter 6, the true distinction between hydrogels and organogels is the magnitudes of the factors that govern the aggregation of the gelator molecules in the two types of media. The importance of another factor, molecular chirality, is evidenced by the absence of a twist in the ribbons made from the racemic modification of the dodecanoylserine; the ribbons from the L-enantiomer are twisted and have a regular pitch whose magnitude depends on additional factors related to the aggregation and growth steps (as well as the properties of the solvent).
Figure 1.3 L-Dodecanoylserine solvent-dependent aggregation routes to tubules. Reprinted with permission from C. Boettcher, B. Schade and J.-H. Fuhrhop, Langmuir, 2001, 17, 873.6 Copyright (2001) American Chemical Society.
Thus, chirality at the molecular level can be transmitted in some (but not all) cases to much larger objects. However, ribbons made from the association of achiral molecules can be twisted as well if such changes reduce interfacial energies with the solvent.9 The parameters associated with how and when the chirality of single molecules is manifested in their assemblies has been investigated in depth by Shimizu, Masuda, and their coworkers in the closure of ribbons into nanotubes10 and theoretically and experimentally by Selinger et al.11,12 In that regard, Oda et al. have examined in detail the parameters controlling the degree and sense of twist of ribbons comprised of nonchiral cationic gemini surfactants, such as one with the formula C2H4-1,2-((CH3)2N+C16H33)2 (16-2-16), and both the chiral enantiomeric and meso forms of tartrate as the counterion.13,14 A graphic presentation of how the nature of the tartrate (and other conditions described in the references cited) can influence the aggregates is shown in Figure 1.4. Formation of the objects in the figure occurs when the enantiomeric excess (ee) of the tartrate is as low as 80%. Below ee values of 60%, only twisted ribbons can be formed; they do not close to form tubules. Also, as expected, the pitch of the ribbons approaches infinity (i.e., flat ribbons) as the ee approaches zero. For enantiomericall...