
eBook - ePub
Biofouling of Membrane Systems
- 330 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Biofouling of Membrane Systems
About this book
Because of the uneven distribution of fresh water in time and space and the increasing human population, a large number of regions are experiencing water scarcity and stress. Membrane-based desalination technologies like reverse osmosis have the potential to solve the fresh water crisis in coastal areas. However, in many cases membrane
performance is restricted by biofouling.
Biofouling of Membrane Systems gives a comprehensive overview on the state of the art strategies to control biofouling in spiral wound reverse osmosis membrane systems and point to possible future research directions. Despite the fact that much research and development has been done to overcome biofouling in spiral wound membrane systems used for water treatment, biofouling is still a major practical problem causing performance decline and increased energy demand. Biofouling of Membrane Systems is divided into three sections including modelling and numerical analysis, non-destructive characterization and feed spacer geometry optimization. It focuses on the development of biomass in the feed channel of the membrane module and its effect on pressure drop
and hydrodynamics.
This book can be used to develop an integral strategy to control biofouling in spiral wound membrane systems. An overview of several potential complementary approaches to solve biofouling is given and an integrated approach for biofouling control and feed spacer design is proposed.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Biofouling of Membrane Systems by Szilárd Bucs,Joop Kruithof,Mark C. M. van Loosdrecht,Johannes Simon Vrouwenvelder in PDF and/or ePUB format, as well as other popular books in Biological Sciences & Applied Sciences. We have over one million books available in our catalogue for you to explore.
Information
© IWA Publishing 2018. Szilárd Bucs, Joop Kruithof, Mark van Loosdrecht and Johannes Vrouwenvelder Biofouling of Membrane Systems DOI: 10.2166/9781780409597_0001
Chapter 1
New approaches to characterizing and understanding biofouling of spiral wound membrane systems
INTRODUCTION
Surfaces in contact with water will inevitably be colonized by bacteria forming a biofilm layer. Membrane units are therefore intrinsically excellent systems for bacteria to colonize. Not surprisingly, biofouling is an important problem in the application of membrane filtration processes for water treatment. Biofouling has been, and still is, an important research topic which until now has not been solved. This is largely due to the very complex nature of the problem involving physical, chemical and biological aspects in a complex geometry. Partly because of the complexity of the problem, biofouling studies have a rather anecdotal nature and the information obtained from pilot and full scale installations is rather anecdotal. Biofilm growth occurs on each surface in contact with water, even in ultrapure water systems (Chicote et al., 2004). In principle, for membrane filtration systems, biofilm growth is not equal to biofouling. Only when biofilm growth leads to a significantly decreased membrane flux, a much increased operational pressure of the system or a decrease in retention properties of the membrane is it called biofouling. In other words, while a biofilm has a clear definition, biofouling is related to operational practises. A further problem is caused by the fact that water quality is not feasible for biofouling characterization. In addition, sampling and analysing membrane units is not easy. Membrane processes are typically black box systems that can only be studied effectively by an autopsy at the end of a filter run. Therefore there is a need for well-defined tools allowing the assessment of the complex interactions occurring in such process units. Finally, biofouling in low pressure membranes (microfiltration (MF), ultrafiltration (UF)) and high pressure membranes (nanofiltration (NF), reverse osmosis (RO)) cannot be directly compared. This is due to the fact that different fouling mechanisms play a dominant role in these systems. One example is the concept of critical flux which is applicable for microfiltration and ultrafiltration (Bacchin et al., 1995; Field et al., 1995; Howell, 1995) but is not relevant for NF and RO systems (Vrouwenvelder et al., 2009b, d). In this contribution we focus on NF and RO-membrane fouling.
BIOFOULING STUDIES
Biofouling control is pursued by: (i) biological pre-treatment removing nutrients (e.g. sand filtration), (ii) applying biocide dosage or UV irradiation (metabolic inactivation of bacteria), (iii) membrane and spacer modification; and (iv) chemical cleaning. Development of membranes materials or coatings which prevent adhesion is one general advocated method to prevent biofouling without the need of applying toxic chemicals. In view of the wide range of possibilities of microorganisms to anchor themselves to surfaces, these approaches to avoid this adhesion do not lead to success. Protection against overgrowth by microorganisms in higher organisms (animals and plants), is mainly obtained by continuous renewal of epithelium cells or, e.g. applying strong wax layers or structures (e.g. lotus leaves). The first is difficult in non-living systems, while the latter is usually not a realistic option. Lotus leaves have a very strong hydrophobic nanostructure which makes them effectively dry and not in contact with the water, protecting them against microbial colonization. This characteristic is sometimes suggested for membrane modification (Marmur, 2006), however the hydrophobic nature makes the membranes unsuitable for water permeation (except as a vapour). Surface modification by hydrophilic groups or polymer brushes is also suggested for minimization of microbial adhesion. The effects of these modifications are usually investigated in short term studies. These studies focus on initial adhesion, which is not representative for biofilm formation (Gjaltema et al., 1997). In general, adhesion is influenced by physico-chemical interactions (for instance electrostatic forces, hydrophobicity, etc.) while biofilm formation is dependent on physical interactions (biofilm strength, shear forces, attachment surface roughness). A low adhesion surface might delay biofilm formation, but not prevent it. Moreover in practical systems a surface gets quickly covered by a conditioning layer from organic molecules in the treated water, masking the surface modification effect. In order to study biofouling a ‘scaled-down’ monitor representative of actual membrane processes is needed in order to be able to do controlled experiments and analyse the system. In essence, for spiral wound NF and RO membrane systems this can be done by a system that represents the geometry of one feed channel with identical geometry and flow rates. The recently developed biofouling monitor (Vrouwenvelder et al., 2006) has such properties. This system makes the biofouling more experimentally accessible. Nevertheless in such experimental systems the complex interaction of processes in, for instance, a biofilm in a NF /RO module are still difficult to assess. It is difficult to change just one parameter at a time; an increased flow rate means more shear, more mass transfer and a different biofilm geometry, all affecting the impact of the biofilm on the filtration processes. In-situ visualization methods such as nuclear magnetic resonance (NMR) imaging (Graf von der Schulenburg et al., 2008; Vrouwenvelder et al., 2009a) or CT-scanning of full membrane modules allows us to assess how processes at the micrometre scale influence the macroscopic behaviour of the membrane processes. Mathematical simulation models can help to quantitatively assess the interaction of the many different processes (flow, diffusion, permeation, microbial growth, etc.), which individually are well known (Picioreanu et al., 2009; Radu et al., 2010). This combination of experimental and modelling approaches has been shown to be a fruitful approach leading to better understanding of biofouling, as discussed below. The main items will be addressed in the following sections
RECENT MICROBIOLOGICAL RESEARCH INTO CHARACTERIZING BIOFOULING
Using flow cells allows regular sampling and analysis of the microbial community that leads to biofouling. (Bereschenko et al., 2010) indicated that in a fresh water RO module, first colonization likely occurs by sphingomonas-type bacteria, a bacterium hitherto not studied in this context. These organisms are well adapted to oligotrophic environments and very rapidly form a gelatinous monolayer on a fresh membrane. These organisms have twitching and swarming motilities, which allow them to rapidly colonize a fresh membrane (Bereschenko et al., 2011; Pang et al., 2005). This thin layer does not directly influence the membrane performance in fresh water systems, but when further colonized by a thicker layer of other microorganisms, fouling occurs. Sphingomonads form in a developed fouling layer only a minor fraction of the microbial population and will not easily be recognized as important, but as they may form the connection between the membrane and the rest of the fouling layer they could form a good target for microbial control. In this respect it is also relevant to realize that these organisms generally make a different extracellular polymeric substances (EPS) than most other bacteria. Herzber et al. (2007) concluded that the RO membrane selects for a specific microbial community. Indeed better characterization of the organisms involved might open the way to directed control of the specific bacteria and their EPS involved. Chemical cleaning leads to a reduction of the pressure over the feed spacer in an RO module. However after chemical cleaning still a major fraction of the biofilm is present as dead cell material in a gel matrix (Figure 1.1). Likely pieces of biofilm subjected to large shear have been detached, resulting in less pressure drop, however the majority of the biofilm is still present (Bereschenko et al., 2011). Molecular genomic studies showed that after a chemical cleaning the sphingomonads (living in the deeper layer of the fouling layer and thereby more protected from cleaning) quickly regrow, (Figure 1.2) likely on the cell mass. Within a short time the fouling is established again.
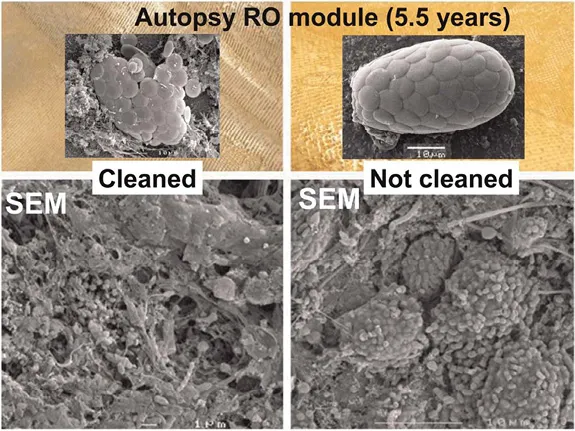
Figure 1.1 EM pictures from full scale RO membranes after 5 years of operation. Left side was cleaned, right membrane module was not cleaned. Both show copious amounts of biomass, although the left side of the biomass shows a more deteriorated image.
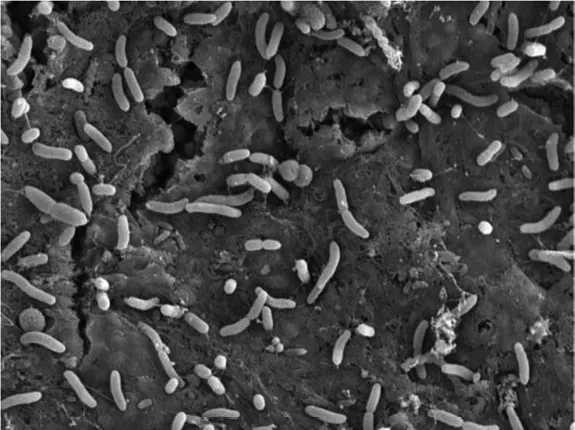
Figure 1.2 Rapid recolonization of a dead ‘cleaned’ fouling layer by sphingomonads.
FLOW CELL STUDIES
Biofilm morphology (and the related impact on pressure drop) is influenced not only by substrate load but also by linear velocity, substrate concentration and fluid shear on the biofilm (Vrouwenvelder et al., 2009b), showing the complexity in interpreting pressure drop measurements as signal for microbial growth in membrane modules. In practice, applied cleanings usually inactivate but do not remove (most of) the accumulated biomass from the RO/NF membrane module, which is the reason why most cleanings are not effective for biofouling control. The effect of chemical cleaning on biofouled RO systems has been studied with the in-situ MRI technique (Creber et al., 2010b).
Chemical cleaning at an early stage of biofouling was more efficient in removing biomass than cleanings performed at a later stage. Preventive cleaning is probably preferred over curative cleaning. The effect of substrate concentration, linear flow velocity, substrate load and flow direction on pressure drop development and biofilm accumulation was investigated in membrane fouling simulators (Vrouwenvelder et al., 2009b) The pressure drop increase was related to the amount of accumulated biomass and linear flow velocity. Biomass accumulation was related to the substrate load. In RO and NF membrane elements, at linear flow velocities applied in practice, voluminous and filamentous biofilm structures were developed in the feed spacer channel, causing a significant increase in feed channel pressure drop. The amount of accumulated biomass was independent of the applied shear, but was depending on the substrate load. A high shear force resulted in a more compact and less filamentous biofilm structure compared with a low shear force, causing a lower feed channel pressure drop increase. This is in accordance with observation in other biofilm systems. A biofilm grown at low shear is however easier to remove by water flushing compared with a biofilm grown at high shear. The flow regime can be used to manipulate the biofilm morphology, enabling new approaches to control biofouling. Operating membranes at low flow velocity reduces the effect of biomass accumulation on feed channel pressure drop/increase (Vrouwenvelder et al., 2009b). Membrane systems could be operated at an optimal feed channel pressure, tolerating biomass accumulation. In other words: a high biomass concentration causing a low feed channel pressure drop increase only is not a problem. Therefore, from a practical point of view the focus of research should be restricting the feed channel pressure drop and the feed channel pressure drop/increase rather than biomass accumulation. The Opti- flux concept (van Paassen et al., 2005) reduces the operational costs of membrane filtration by applying fewer membrane modules in a pressure vessel than commonly applied (i.e. three modules instead of up to eight). The operational cost reduction is based on lower energy consumption. In addition, a lower flow velocity will impact the effect of the biomass amount on the feed channel pressure drop, so the Optiflux concept may reduce the feed channel pressure drop increase caused by biofouling, reducing the membrane operational costs even further. Biomass accumulation is reduced at the location where i...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Contents
- Preface
- List of authors
- Chapter 1: New approaches to characterizing and understanding biofouling of spiral wound membrane systems
- Chapter 2: Effect of flow velocity, substrate concentration and hydraulic cleaning on biofouling of reverse osmosis feed channels
- Chapter 3: Modelling the effect of biofilm formation on reverse osmosis performance: Flux, feed channel pressure drop and solute passage
- Chapter 4: Combined biofouling and scaling in membrane feed channels: A new modelling approach
- Chapter 5: Effect of different commercial feed spacers on biofouling of reverse osmosis membrane systems: A numerical study
- Chapter 6: In-situ biofilm characterization in membrane systems using Optical Coherence Tomography: Formation, structure, detachment and impact of flux change
- Chapter 7: Early non-destructive biofouling detection and spatial distribution: Application of oxygen sensing optodes
- Chapter 8: Chemical cleaning of biofouling in reverse osmosis membranes evaluated using magnetic resonance imaging
- Chapter 9: Early non-destructive biofouling detection in spiral wound RO membranes using a mobile earth’s field NMR
- Chapter 10: Experimental and numerical characterization of the water flow in spacer-filled channels of spiral-wound membranes
- Chapter 11: Spacer geometry and particle deposition in spiral wound membrane feed channels
- Chapter 12: Characterization of feed channel spacer performance using geometries obtained by X-ray computed tomography
- Chapter 13: Development and characterization of 3D-printed feed spacers for spiral wound membrane systems
- Chapter 14: Strategies for biofouling mitigation in spiral wound membrane systems
- References
- Index