
- 361 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
Biomedical Chemistry provides readers with an understanding of how fundamental chemical concepts are used to combat some diseases. The authors explain the interdisciplinary relationship of chemistry with biology, physics, pharmacy and medicine. The results of chemical research can be applied to understand chemical processes in cells and in the body, and new methods for drug transportation. Also, basic chemical ideas and determination of disease etiology are approached by developing techniques to ensure optimum interaction between drugs and human cells. This Book is an excellent resource for students and researchers in health-related fields with frontier topics in medicinal and pharmaceutical chemistry, organic chemistry and biochemistry.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Section 1: Chemical Principles in Drug Design and Discovery
Diana C. G. A. Pinto, João M. P. Pereira, Artur M. S. Silva*
1.1 Functional Groups of Biomolecules and their Reactions
Abstract: This chapter starts with a general introduction on some concepts needed to understand the reactivity of organic functional groups. Elementary reaction mechanisms are then presented according to their functionality with relevant biological examples. These reactions explain the vast majority of transformations involving biomolecules. Finally, two examples on the application of the presented concepts are given, namely the metabolism of fatty acids and reactivity of penicillin. Both of these examples call for various types of reactions showing the diversity and simplicity of biological transformations when analysed step by step.
1.1.1 Functional Groups in Biological Systems
The main definition of a functional group in organic chemistry books is as a chemically reactive group of atoms within a molecule that contribute to its characteristic reactivity. Functionality is usually regarded as “implying the presence of heteroatoms and/or unsaturation, but it would not be helpful to attempt to define precisely the limits of application of the term” (IUPAC, Commission on Nomenclature of Organic Chemistry, 1993).
Functional group reactivity may be changed by the presence of other neighbouring functional groups but usually behaves uniformly in every molecule where it can be found. There are several common functional groups that are related to families of organic compounds according to their structural features. However, from those functional groups only a few are found in biological systems (Table 1.1.1). The types of bonding found in these functional groups may be explained by the existence of various hybrid atomic orbitals of the carbon atom created from combination of the one 2s and the three 2p orbitals (Table 1.1.2).
Table 1.1.1: Common functional groups present in biomolecules. In parentheses are the names of the families of compounds, where the group has the highest priority in the compound.
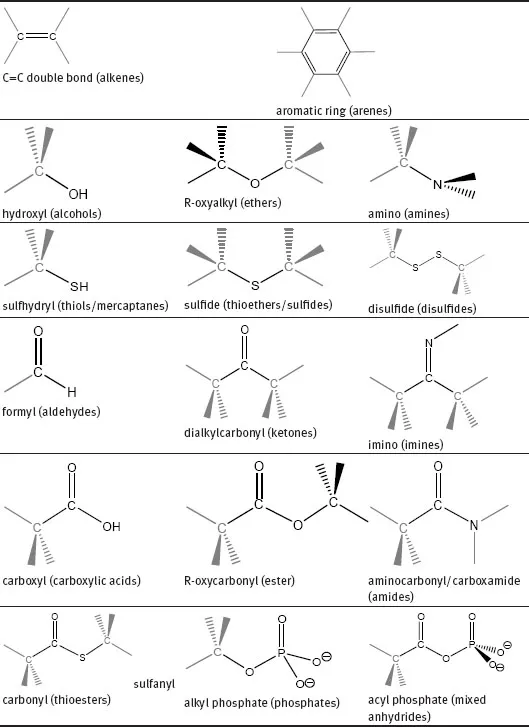
Table 1.1.2: Possible orbital combinations in the carbon atom, their spatial configuration and geometry with inter-bond angles.
| Combination | Hybrid result | Geometry |
1 x 2s + 3 x 2p | 4 x sp3 | 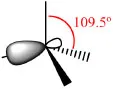 |
1 x 2s + 2 x 2p | 3 x sp2 | 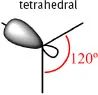 |
1 x 2s + 2 x 2p | 2 x sp | 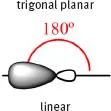 |
All of the hybrid orbitals may be used to form σ (sigma) molecular orbitals by fusion with s or with other hybrid orbitals from other atoms (according to Molecular Orbital Theory). If there are remaining 2p orbitals in the carbon atoms (in the case of sp2 and sp hybrid orbitals) they are used to form π (pi) molecular orbitals by lateral combination with other adjacent 2p orbitals (Fig. 1.1.1). A simple bond is formed with a single σ-bond; the double bond is formed with a σ-bond and a π-bond; the triple bond is formed with a σ-bond and two π-bonds.
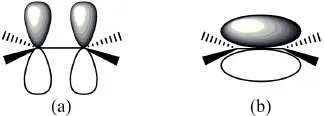
Figure 1.1.1: A simplified representation of the spatial distribution of two adjacent p orbitals (a) and their combination into a π bond (b) between two sp2 carbon atoms. The π-bonds in a triple bond would be along orthogonal planes to each other.
These hybridisations have several consequences, such as the electron density of a π-bond lying above and below the plane of the bonding atoms (Fig. 1.1.1), resulting in greater exposure for a reaction. Simultaneously, with the increasing s character of the hybrid orbital:
–the formed bond length decreases;
–the polarity of a C-H bond increases;
–breaking a bond between carbon and a more electronegative atom is more difficult (e.g. the C-O bond in isopropanol is easier to cleave than the C-O bond in isopropenol).
The electronegativity of an element can also be an important factor to explain some functional groups reactivity. For instance, alcohols, ethers, amines, thiols, sulfides, disulfides and phosphates (Table 1.1.1) all have a carbon forming a single bond with a more electronegative atom, causing the carbon to bear a partial positive charge (δ+). These modifications affect both the σ- and π-bonds, although in the case of some π-bonds the resonance effect should also be considered. The carbonyl group can be classically treated as a resonance hybrid represented by two resonance structures (Scheme 1.1.1), which contributes to the reactivity of the compounds that have this functional group (aldehydes, ketones, carboxylic acids, esters, thioesters, amides, acyl phosphates). Resonance is possible whenever movement of electrons are allowed within the same molecule without movement of atoms.
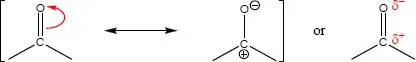
Scheme 1.1.1: Carbonyl group resonance structures and equivalent notation, explicitly showing the bond polarity with partial charges.
1.1.2 Acids and Bases Versus Electrophiles and Nucleophiles
Acids and bases are very important in biological transformations, as most require some form of acid or basic catalysis to occur. The simplest acid-base theory is the Brønsted-Lowry theory, which states that acids are molecules that donate protons (hydrogen ions, H+) and bases/alkalis are molecules that accept protons. For example, a carboxylic acid can donate a proton to a base, such as an amine, in a reversible proton-transfer reaction (Scheme 1.1.2).
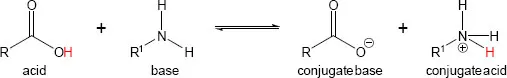
Scheme 1.1.2: Example of a proton transfer reaction. This specific reaction explains why it is difficult for condensations to happen directly between an amine and a carboxylic acid, as the non-ionic forms of these molecules are more reactive (Chapter 1.1.4.5).
Acids can differ in their ability to donate protons, being classified as strong or weak according to the extent of deprotonation. A strong acid will have a stable conjugate base (or weak conjugate base), resulting in ready donation of a proton. Table 1.1.3 lists the acidity of some typical functional groups (water and ammonium acidity are also given for comparison). The acidity is measured by the acidity constant, Ka or by its pKa (Scheme 1.1.3), where a stronger acid has a smaller pKa and a weaker acid has a larger pKa. The same approach can be applied to bases and their strength.
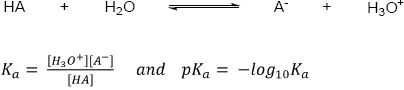
Scheme 1.1.3: Acidity constant and pKa.
The problem with the Brönsted/Lowry definition is that it only covers the compounds that donate or accept protons. The more general and widely used model is the Lewis definition. A Lewis acid is a molecule that accepts a pair of electrons and a Lewis base is a molecule that donates a pair of electrons. To accept electrons, a Lewis acid must have a vacant low-energy orbital. As a consequ...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright
- Contents
- Preface
- Section 1: Chemical Principles in Drug Design and Discovery
- Section 2: Chemical Basis of Drug Action and Diseases
- Section 3: Strategies to Develop New and Better Drugs
- Index
- BackCover
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Biomedical Chemistry by Nuno Vale in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Biochemistry. We have over one million books available in our catalogue for you to explore.