
Handbook Of Immunological Properties Of Engineered Nanomaterials
- 720 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Handbook Of Immunological Properties Of Engineered Nanomaterials
About this book
The Handbook of Immunological Properties of Engineered Nanomaterials provides a comprehensive overview of the current literature, methodologies, and translational and regulatory considerations in the field of nanoimmunotoxicology. The main subject is the immunological properties of engineered nanomaterials. Focus areas include interactions between engineered nanomaterials and red blood cells, platelets, endothelial cells, professional phagocytes, T cells, B cells, dendritic cells, complement and coagulation systems, and plasma proteins, with discussions on nanoparticle sterility and sterilization. Each chapter presents a broad literature review of the given focus area, describes protocols and resources available to support research in the individual focus areas, highlights challenges, and outlines unanswered questions and future directions. In addition, the Handbook includes an overview of and serves a guide to the physicochemical characterization of engineered nanomaterials essential to conducting meaningful immunological studies of nanoparticles. Regulations related to immunotoxicity testing of materials prior to their translation into the clinic are also reviewed.
The Handbook is written by top experts in the field of nanomedicine, nanotechnology, and translational bionanotechnology, representing academia, government, industry, and consulting organizations, and regulatory agencies. The Handbook is designed to serve as a textbook for students, a practical guide for research laboratories, and an informational resource for scientific consultants, reviewers, and policy makers. It is written such that both experts and beginners will find the information highly useful and applicable.
Contents:
- Immunological Properties of Engineered Nanomaterials: An Introduction (Marina A Dobrovolskaia and Scott E McNeil)
- Importance of Physicochemical Characterization Prior to Immunological Studies (Jeffrey D Clogston and Anil K Patri)
- Impact of Nanoparticle Sterilization on Analytical Characterization (Nanda Subbarao)
- Endotoxin and Engineered Nanomaterials (Marina A Dobrovolskaia and Scott E McNeil)
- Surface Adsorbates on Nanomaterials and Their Possible Roles in Host Inflammatory and Toxicological Processing (Clinton F Jones, David G Castner, and David W Grainger)
- Nanoparticle Interaction with Plasma Proteins as It Relates to Biodistribution (Lennart Treuel and G Ulrich Nienhaus)
- The Effects of Engineered Nanomaterials on Erythrocytes (Bridget Wildt, Richard A Malinauskas, and Ronald P Brown)
- The Effects of Engineered Nanomaterials on Cultured Endothelial Cells (Jan Simak)
- The Effect of Engineered Nanomaterials on the Plasma Coagulation System (Jan Simak)
- The Effects of Engineered Nanomaterials on Platelets (Jan Simak)
- Complement Activation (Carolina Salvador-Morales and Robert B Sim)
- Bidirectional Interaction Between Nanoparticles and Cells of the Mononuclear Phagocytic System (Whitney P Caron, Sumit Rawal, Gina Song, Parag Kumar, John C Lay, and William C Zamboni)
- The Effects of Nanoparticles on Dendritic Cells (Valentyna Fesenkova)
- The Effects of Nanoparticles on Bone Marrow Cells (Ekaterina Dadachova)
- Nanoparticles, Immunomodulation and Vaccine Delivery (Sue D Xiang, Martina Fuchsberger, Tanya De L Karlson, Charles L Hardy, Cordelia Selomulya and Magdalena Plebanski)
- Nanoparticles as Drug Delivery Vehicles for the Therapy of Inflammatory Disorders (Deepthy Menon, J Gopikrishna, Dhanya Narayanan, and Shantikumar V Nair)
- Nanostructures and Allergy (Silvia Lorenzo-Abalde and África González-Fernández)
- Nanoparticles and Antigenicity (Marina A Dobrovolskaia)
- In Vitro Assays for Monitoring Nanoparticle Interaction with Components of the Immune System (Marina A Dobrovolskaia and Scott E McNeil)
- Evaluating the Adverse Effects of Nanomaterials on the Immune System with Animal Models (Matthew J Smith, Colleen E McLoughlin, Kimber L White, Jr, and Dori R Germolec)
- Immunotoxicity Testing for Drug–Nanoparticle Conjugates: Regulatory Considerations (Simona Bancos, Katherine M Tyner, and James L Weaver)
Readership: Researchers, academics, undergraduates and graduates in toxicology, immunotoxicology and nanomedicine, and industry (small and mid biotech companies and big pharmaceutical companies), as well as regulatory agencies (EPA, FDA) and physicians.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Chapter 1
Immunological Properties of Engineered
Nanomaterials: An Introduction
Advanced Technology Program, SAIC-Frederick, Inc.
NCI-Frederick, Frederick, MD 21702, USA
1. Introduction
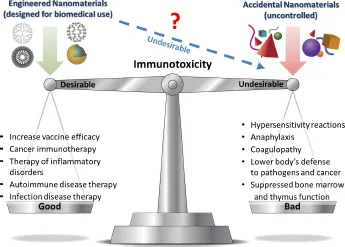
2. Interaction with Blood Components
2.1. Hemolysis
Table of contents
- Cover
- Half Title
- dedication
- Title
- Copyright
- contents
- Preface
- List of Contributors
- Chapter 1
- Chapter 2
- Chapter 3
- Chapter 4
- Chapter 5
- Chapter 6
- Chapter 7
- Chapter 8
- Chapter 9
- Chapter 10
- Chapter 11
- Chapter 12
- Chapter 13
- Chapter 14
- Chapter 15
- Chapter 16
- Chapter 17
- Chapter 18
- Chapter 19
- Chapter 20
- Chapter 21
- Index