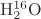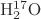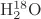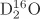![]()
Chapter 1
Molecular Structures of Water and Its Features
This chapter answers the question “what is water?”. Through wide researches, we confirm that water is not a uniform liquid composed only of free water molecules, but a non-uniform liquid, which is composed of free water molecules, linear and ring hydrogen-bonded chains and clusters of water molecules with different configurations and weights. A basic feature of its structure is that the non-uniformity of composition varies slowly with changing temperature of water, rather than remaining constant. The proposed new structure of water is confirmed by the experiments of infrared spectra of absorption, the experimental results of energy-spectrum of vibration and self-assembly and numerical simulation of its molecules as well as complicated phase diagram, which are systematically studied and described in detail in this chapter. The singular and anomalous properties of water arising due to its complicated molecular structure will be discussed in Chapters 2, 3 and 4, respectively.
1.1.The New Theory of Molecular Structure in Water and Experimental Evidences
1.1.1.The Structure of Water Molecule and Its Polarization Feature
As it is known, water molecule is composed of two hydrogen atoms and one oxygen atom. This structure came from the experiments of Cavendish and Lavoisier in the 1780s, who established that water is composed of hydrogen and oxygen. Even though their data were sufficient to prove that two volumes of hydrogen can combine with one volume of oxygen, they did not point out clearly the structure of water molecule, which was left to Gay-Lussac and Humboldt to make this discovery in 1805. Dumas, in 1842, found that the ratio of the combining weights of hydrogen and oxygen in water is nearly 2 to 16 [1]. A lot of people studied molecular structure of water, but it is not clear as yet [2–10]. Clearly, the electronic structure of water molecules can be denoted by 1sO2.00 2sO1.82 2pxO1.50 2pzO1.12 2pyO2.00 1sH10.78 1sH20.78 [11], however it appears that the 2s orbital may be effectively unhybridized with the bond angle expanded from the expected angle of 90° due to steric and ionic repulsions between the partially-positively charged hydrogen atoms (as proposed by Pauling over 50 years ago [12]). Therefore, the molecular orbitals of water appears as (1a1)2(2a1)2(1b2)2(3a1)2(1b1)2. Shown opposite is the electrostatic potential associated with the water structure. Although the lone pairs of electrons do not appear to give distinctly directed electron density in isolated molecules, there are minima in the electrostatic potential approximately in the expected positions.
Water molecules are V-shaped with molecular formula H2O, which is symmetric (point group C2ν) with two mirror planes of symmetry and a two-fold rotation axis. The hydrogen atoms may possess parallel or antiparallel nuclear spin [10–12]. In the structure, the approximately 16-fold difference in mass between the light atoms (H) and a relatively heavy atom (O) is the reason behind its ease of rotation and the significant relative movements of the hydrogen nuclei, which are in constant and significant relative movements. This confirms that the structure of water molecule is usually permanent.
In the liquid state, in spite of 80% of the electrons being concerned with bonding, the three atoms do not stay together as the hydrogen atoms are constantly exchanging between water molecules due to protonation/deprotonation processes. Both acids and bases can catalyze this exchange and even when at its slowest (at pH 7), the average time for the atoms in an H2O molecule to stay together is only about a millisecond. The water molecule is often described as having four, approximately tetrahedrally arranged, sp3-hybridized electron pairs in the liquid state, two of which are associated with hydrogen atoms leaving the two remaining lone pairs. In a perfect tetrahedral arrangement the bond–bond, bond–lone pair and lone pair–lone pair angles would all be 109.47° and such tetrahedral bonding patterns are found in condensed phases such as hexagonal ice. However, Grabowski [13] found from the study of the hydrogen bonding strength in water by ab initio method that ab initio calculations on isolated molecules cannot confirm the presence of significantly directed electron density where lone pairs are expected, where the negative charge is more evenly smeared out along the line between where these lone pairs would have been expected, and lies closer to the center of the O-atom than the centers of positive charge on the hydrogen atoms. Therefore, there is no apparent consensus of opinion [14] for the descriptions of substantial sp3-hybridized lone pairs in the isolated water molecule, perhaps an sp2-hybridized structure (plus a pz orbital) is possible. This rationalizes the formation of trigonal hydrogen bonding (almost planar) that can be found around some restricted sites in the hydration of proteins and where the numbers of hydrogen bond donors and acceptors are unequal.
Parameters using
ab initio calculations are shown right. For an isolated
,
or
molecule, the exact calculated O–H length is 0.0957854 and the H–O–H angle is 104.500° (
, 0.0957835 nm, 104.490°)
[15], [O–H length is 0.0991 nm and H–O–H angle is 105.5°
[16]]. But diffraction studies of various combinations O–H length 0.101 nm, O–D length 0.098 nm
[17]; O–H length 0.0990 nm, O–D length 0.0985 nm
[18]; O–D length 0.0970 nm, D–O–D angle 106°
[19] suggest slightly greater values, which are caused by the hydrogen bonding weakening the covalent bonding and reducing the repulsion between the electron orbitals. These bond lengths and angles are likely to change, due to polarization shifts, in different hydrogen-bonded environments and when the water molecules are bound to solutes and ions. Commonly used molecular models use O–H lengths between 0.0957 nm and 0.100 nm and H–O–H angles of 104.52° to 109.5°. The charge distribution depends significantly on the atomic geometry and the method for its calculation but is likely to be about –0.7e on the O-atom (with the equal but opposite positive charge equally divided between the H-atoms) for the isolated molecule
[20]. Yet, the experimental values for gaseous water molecule are O–H length 0.095718 nm, H–O–H angle 104.474°
[21], which are not maintained in liquid water.
The mean van der Waals diameter of water molecule was 0.282 nm, which is identical with that of isoelectronic neon [22, 23]. Molecular model values and intermediate peak radial distribution data indicate however that it is somewhat greater (∼0.32 nm) [23]. The molecule is clearly not spherical, however, with about a ±5% variation in van der Waals diameter dependent on the axis chosen; approximately tetrahedrally placed slight indentations being apparently opposite to the (putative) electron pa...