
Endohedral Fullerenes: From Fundamentals To Applications
From Fundamentals to Applications
- 448 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Endohedral Fullerenes: From Fundamentals To Applications
From Fundamentals to Applications
About this book
Endohedral fullerenes represent a novel family of carbon nanostructures, which are characterized by a robust fullerene cage with atoms, ions, or clusters trapped in its interior. Since the first separation of the endohedral metallofullerene La@C 82 in 1991, a large variety of endohedral structures have been isolated and their endohedral nature has been proved by experimental studies. Within the past two decades, the world of endohedral fullerenes was significantly enlarged by the clusterfullerenes and the new carbon cages including non-IPR (IPR=isolated pentagon rule) structures. Resulting from the charge transfer from the encaged species to the fullerene cage, endohedral fullerenes hold a lot of fascinating properties inaccessible by the empty fullerenes, and consequently promise potential applications in biomedicine, molecular electronics and photonics etc.
The book provides a comprehensive overview of endohedral fullerenes focused on the new advances in the past decade, including its fundamentals (structures), synthesis, isolation, characterization, properties, functionalization as well as the applications, thus representing the most updated and broad review of this exciting field.
Contents:
- The Early Days of Metallofullerene Research (Hisanori Shinohara)
- Synthesis and Isolation of Endohedral Fullerenes — A General Review (Fupin Liu, Jian Guan, Tao Wei, Song Wang and Shangfeng Yang)
- Crystallographic Study of Endohedral Metallofullerenes (Yun-Peng Xie, Shasha Zhao and Xing Lu)
- Metal Nitride Clusterfullerenes — New Advances and Challenges (Tao Wei, Song Wang, Fupin Liu, Jian Guan, Alexey A Popov, Lothar Dunsch and Shangfeng Yang)
- Metal Carbide Clusterfullerenes (Taishan Wang and Chunru Wang)
- The Discovery of Non-IPR Fullerenes (Wei Xu, Chunying Shu and Chunru Wang)
- Metal Oxide Clusterfullerenes (Steven Stevenson)
- Nitrogen Atom-Based Endohedral Fullerenes and Potential Applications (B J Farrington and K Porfyrakis)
- Noble-Gas Fullerenes (R James Cross, Jr)
- Electrochemical Properties of Endohedral Metallofullerenes (Luis Echegoyen, Frederic Melin and Manuel N Chaur)
- Chemical Functionalization of Endohedral Metallofullerenes (Yutaka Maeda)
- Computational Studies of Endohedral Fullerenes: Bonding, Isomerism, Internal Dynamics, Spectroscopy, and Chemical Reactivity (Alexey A Popov)
- Biomedical Applications of Trimetallic Nitride Endohedral Metallofullerenes (Jianyuan Zhang, Boris M Kiselev, Youqing Ye and Harry C Dorn)
- Higher LUMO Level Endohedral Fullerene and Fullerene Bisadduct Acceptors for Polymer Solar Cells (Yongfang Li)
Readership: Advanced undergraduates and graduate students, scientists in Chemistry, Physics, and Materials Science, researchers and professionals in the fields of fullerenes and all-carbon nanomaterials, and the general public.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
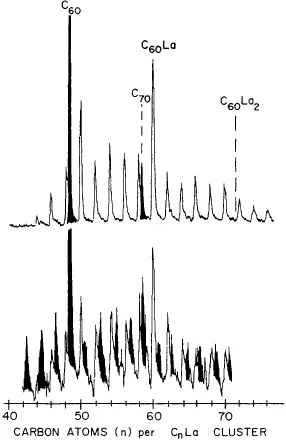
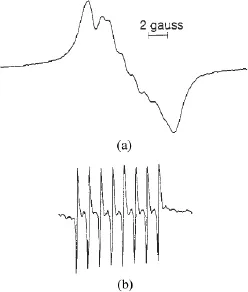
Table of contents
- Cover
- Halftitle
- Title
- Copyright
- Dedication
- Foreword
- Contents
- Chapter 1 The Early Days of Metallofullerene Research
- Chapter 2 Synthesis and Isolation of Endohedral Fullerenes — A General Review
- Chapter 3 Crystallographic Study of Endohedral Metallofullerenes
- Chapter 4 Metal Nitride Clusterfullerenes — New Advances and Challenges
- Chapter 5 Metal Carbide Clusterfullerenes
- Chapter 6 The Discovery of Non-IPR Fullerenes
- Chapter 7 Metal Oxide Clusterfullerenes
- Chapter 8 Nitrogen Atom-Based Endohedral Fullerenesand Potential Applications
- Chapter 9 Noble-Gas Fullerenes
- Chapter 10 Electrochemical Properties of Endohedral Metallofullerenes
- Chapter 11 Chemical Functionalization of Endohedral Metallofullerenes
- Chapter 12 Computational Studies of Endohedral Fullerenes: Bonding, Isomerism, Internal Dynamics, Spectroscopy, and Chemical Reactivity
- Chapter 13 Biomedical Applications of Trimetallic Nitride Endohedral Metallofullerenes
- Chapter 14 Higher LUMO Level Endohedral Fullerene and Fullerene Bisadduct Acceptors for Polymer Solar Cells
- Index