
Understanding Basic Chemistry Through Problem Solving
The Learner's Approach
- 468 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Understanding Basic Chemistry Through Problem Solving
The Learner's Approach
About this book
Written for students taking either the University of Cambridge O-level examinations or the GCSE examinations, this guidebook covers essential topics and concepts under stipulated chemistry syllabi. The topics are explored through an explanatory and inquiry-based approach. They are interrelated and easy to understand, with succinct explanations/examples being included, especially on areas that students frequently find difficult. Topics address the whys and hows behind key concepts to be mastered, so that the concepts are made understandable and intuitive for students. The focus is on conceptual learning so as to equip students with knowledge for critical learning and problem solving.
The authors have also retained the popular discourse feature from their previous four books — Understanding Advanced Physical Inorganic Chemistry, Understanding Advanced Organic and Analytical Chemistry, Understanding Advanced Chemistry Through Problem Solving, and Understanding Basic Chemistry — to help the learners better understand and see for themselves, how the concepts should be applied during problems solving. Based on the Socratic Method, questions are implanted throughout the book to help facilitate the reader's development in forming logical conclusions of concepts and the way they are being applied to explain the problems. In addition, the authors have also included important summaries and concept maps to help the learners to recall, remember, reinforce and apply the fundamental chemical concepts in a simple way.
Through their many years of teaching experiences, the authors have acquired a sound awareness of common students' misconceptions which are relayed through the questions and thus help to reinforce concepts learnt. This book is essential and useful to help students adequately prepare for the high-stake examinations.
Request Inspection Copy
Contents:
- The Particulate Nature of Matter
- Atomic Structure
- Chemical Bonding
- Mole Concept, Formula, and Stoichiometry
- Energy Change and Fuels
- Rate of Chemical Reactions
- Equilibria, Ammonia, and Sulfur
- Acids, Bases, and Salts
- Redox Reactions
- Electric Cells and the Reactivity Series
- Electrolysis
- The Periodic Table
- Metals and Extraction
- Air and the Environment
- Organic Chemistry
- Experimental Chemistry
Readership: Students taking the University of Cambridge O-level examinations or the GCSE examinations.
Key Features:
- The book provides fundamental important scaffolding to aid students to create their own understanding of the chemical concepts in O level chemistry or the GCSE equivalence
- The book encourages critical thinking and meaningful applications, using the basic concepts learnt
- The book guides the students to integrate the various concepts that they have learnt into a coherent and meaningful conceptual network, especially relating to the basic physical chemistry they have learnt
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
| Q | What is a chemical property? |
| Q | So, does that mean that chlorine atom would forever have the same potential to gain electrons irrespective of the type of substances that it reacts with? |
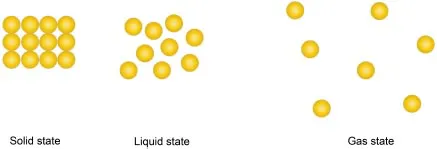
| Q | Why is the compressibility of the solid and liquid so much lower than that for the gas? |
| Q | So, it is actually the weak attractive forces between the particles in the gaseous state that help to “pull” the particles even closer together during compression? |
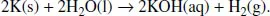
| Q | But when the salt is dissolved, it disappears. So, shouldn’t the change be a chemical one? |
Table of contents
- Cover
- Halftitle
- Title
- Copyright
- Preface
- Acknowledgements
- Content
- Chapter 1 The Particulate Nature of Matter
- Chapter 2 Atomic Structure
- Chapter 3 Chemical Bonding
- Chapter 4 Mole Concept, Formula, and Stoichiometry
- Chapter 5 Energy Change and Fuels
- Chapter 6 Rate of Chemical Reactions
- Chapter 7 Equilibria, Ammonia, and Sulfur
- Chapter 8 Acids, Bases, and Salts
- Chapter 9 Redox Reactions
- Chapter 10 Electric Cells and the Reactivity Series
- Chapter 11 Electrolysis
- Chapter 12 The Periodic Table
- Chapter 13 Metals and Extraction
- Chapter 14 Air and the Environment
- Chapter 15 Organic Chemistry
- Chapter 16 Experimental Chemistry
- Index
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app