
Affinity And Efficacy: The Components Of Drug-receptor Interactions
The Components of Drug-Receptor Interactions
- 752 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Affinity And Efficacy: The Components Of Drug-receptor Interactions
The Components of Drug-Receptor Interactions
About this book
The interaction of a drug with a receptor generates a code of information having components of affinity and efficacy. How this information is translated into a response depends on the unique cells, tissue, organ or system in which the receptor resides. This book describes how to analyze various responses to estimate the affinity and efficacy components of the initial drug–receptor interaction. More specifically, it describes how to measure the affinity and efficacy of drugs through the analysis of single receptor activity, the activation state of a population of receptors, and responses downstream from receptor activation. More light is thrown on ligand-gated ion channels and G protein-coupled receptors in this book.
The topics discussed include radioligand binding, estimation of agonist affinity and efficacy, competitive antagonism, inverse agonism, allosteric agonists and modulators, agonist bias, modulation of pathway selectivity, and the estimation of ligand affinity for active and inactive receptor states. The natural history and structure of ligand-gated ion channels, G proteins, and G protein-coupled receptors are also discussed.
Contents:
- Ligand-Gated Ion Channels:
- Ligand-Gated Ion Channels
- Affinity and Efficacy of Orthosteric Ligands at Ligand-Gated Ion Channels
- Competitive Interactions Between Orthostheric Ligands at Ligand-Gated Ion Channels
- Analysis of Allosteric Interactions at Ligand-Gated Ion Channels
- G Protein-Coupled Receptors:
- G Protein-Coupled Receptors and G Protein and Arrestin Signaling
- Affinity and Efficacy of the Agonist-Receptor- G -Protein Interaction
- Estimating Affinity and Efficacy by Reverse-Engineering the Response-Clamp Analysis
- Analysis of Agonism and Inverse Agonism in Signaling Pathways that Exhibit Constitutive Activity
- Analysis of Ligand Bias in Receptor Signaling through Different G Protein Pathways
- Competitive Interactions Between Orthosteric Ligands in Functional Assays on G Protein-Coupled Receptors
- Analysis of Allosterism in Functional Assays on G Protein-coupled Receptors
- Radioligand Binding:
- Analysis of Drug-receptor Interactions Using Radioligand Binding Assays on G Protein-coupled Receptors
Readership: Postgraduates and researchers in pharmacology and physiology, professionals in the pharmaceutical industry, neuroscience researchers.
Key Features:
- It presents laymen and mathematicians alike with beautiful pieces of mathematics, and studies techniques of both poetry and mathematics that contribute to beauty
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
PART 1
Ligand-Gated Ion Channels
Ligand-Gated Ion Channels | 1 |
Nicotinic Acetylcholine Receptors
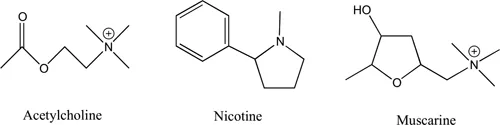
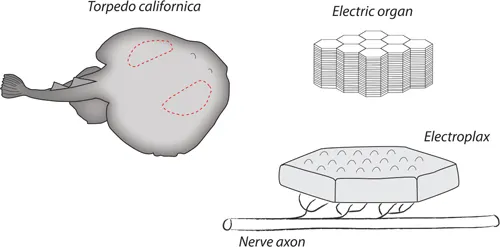
Table of contents
- Cover Page
- Title
- Copyright
- Dedication
- Contents
- Acknowledgments
- Prologue: Drugs, Receptors and Therapeutic Messages
- Part I: Ligand-Gated Ion Channels
- Part II: G Protein-Coupled Receptors
- Part III: Radioligand Binding
- Index