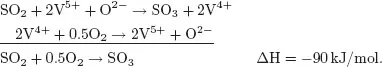![]()
Chapter 1
Introduction
1.1Catalysis and Catalysts
As early as the 18th century it was known that some chemical reactions are accelerated by the addition of compounds and that these compounds can be recovered unchanged after reaction. Some reactions occur only in the presence of such compounds. In 1835, Berzelius introduced the term catalysis to indicate the action of a compound (the catalyst) that increases the rate of a reaction but which does not change during the reaction. Today we know that a catalyst does change when it catalyses a reaction, but it then changes back to its original form. Therefore, it is possible to reuse a catalyst over and over again. The following example of the oxidation of SO2 to SO3 illustrates the catalytic cycle.
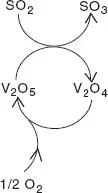
SO2 oxidation proceeds by means of a Mars–van Krevelen mechanism, by which oxygen does not react directly with the reactant. Instead, the reactant is oxidised by a metal oxide (consuming lattice oxygen) and the reduced metal oxide is re-oxidised by oxygen. Thus, in SO3 synthesis, SO2 is first oxidised by V5+ to SO3 and in a second step, V4+ is oxidised back to V5+ by oxygen (catalyst regeneration). The first step is an oxidation of SO2 and a reduction of V5+; in the second step oxygen is reduced and the catalyst is re-oxidised. Thus, after one full catalytic cycle, it seems that the catalyst has not changed. Schematically, this is indicated by a cycle with the catalyst species on the cycle, with reactants entering the cycle and products leaving it (Fig. 1.1), showing that the catalyst can be used an ‘infinite’ number of times.
The oxidation of SO2 to SO3 is an important step in the production of sulphuric acid. After burning sulphur to sulphur dioxide and with the help of a vanadium oxide catalyst, oxidising the SO2 with oxygen to SO3, the resulting SO3 is absorbed into 97–98% H2SO4, to form oleum (H2S2O7). The oleum is then diluted with water to form concentrated sulphuric acid. Sulphuric acid is the number two base chemical, with a worldwide production of 180 million tonnes (MT) in 2004. It is used mainly (∼60%) in the production of ammonium phosphate and ammonium sulphate fertilisers. It is also used to produce chemicals such as hydrochloric acid, nitric acid, sulphate salts, synthetic detergents, dyes, pigments, explosives and drugs. This example shows how important catalysis is in chemical reactions. To be able to perform a reaction, or to perform it under milder conditions than usual, is of enormous importance. It should, therefore, be no surprise that about 90% of all chemicals are made by means of catalysed reactions.
Although Berzelius stated that a catalyst does not change during a catalysed reaction and thus, could be used ad infinitum, in reality things are not that simple. Most catalysts are solids and enable reactions between molecules on their surface, where they occur with greater ease than in the gas or liquid phase. Solid catalysts often become progressively covered with coke, with the result that less catalyst surface is accessible to the reacting molecules and that the activity of the catalyst decreases. When the deactivation of the catalyst is severe, then it is possible to regenerate the catalyst — for instance, by burning off the coke in air and re-reducing the catalyst if required. Another reason for the loss of catalytic activity may be sintering, i.e. growth of the particles that make up the catalyst. Small particles have a large surface area and thus, high catalytic activity. Unfortunately, that also means high surface energy, a thermodynamically instable situation. At an elevated reaction temperature particle growth may take place by diffusion and combination of the particles, or by scission of atoms from the particles and diffusion to other particles. In either case there is a decrease in the catalyst activity, not because of a change in the catalyst structure but because of a loss of accessible surface area. The catalytic reactions are still the same, but the reaction rate has decreased. A better description of a catalyst than given by Berzelius was given by Ostwald (awarded the Nobel Prize in Chemistry in 1909): a catalyst changes the rate but not the chemical equilibrium of a reaction.
1.2Heterogeneous and Homogeneous Catalysis
In heterogeneous catalysis, the catalyst is present as a solid phase and the reactants and products are in a gas or liquid phase. Whereas gas–solid heterogeneous catalysis is often used in the refinery and in the bulk chemicals industry, liquid–solid heterogeneous catalysis is common in the fine chemical industry, where large batch reactors are filled with an organic liquid and a solid catalyst. The V2O5 catalyst for SO2 oxidation (Section 1.1) is a solid catalyst used to convert gaseous SO2 to SO3. In heterogeneous catalysis, the molecules can react only on the surface of the solid catalyst; thus, the goal is to maximise the catalyst surface by means of small catalyst particles, because the specific surface area (the surface area per mass unit) is proportional to 1/R (surface area is proportional to R2 and volume to R3), where R is the diameter of the particles. There is a limit to the size of the catalyst particles, because the space between nano particles is very small, which hinders the flow of the gas or liquid around the catalyst particles (diffusion problems). Furthermore, the smaller the particles, the greater their tendency to sinter into larger particles. To avoid problems with diffusion and sintering, catalyst particles are often put on the surface of other materials, i.e. supports. The particles of the support material are larger than the catalyst particles. This leads to fewer problems with diffusion and the catalyst particles can be placed far enough apart on the support surface so that they do not come into contact with each other and do not sinter. Therefore, the SO2 oxidation catalyst V2O5/SiO2 cons...