
- 224 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
This book summarizes the highlights of our work on the bond polarizability approach to the intensity analysis. The topics covered include surface enhanced Raman scattering, Raman excited virtual states and Raman optical activity (ROA). The first chapt
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Raman Spectroscopy: An Intensity Approach by Guozhen Wu in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Optics & Light. We have over one million books available in our catalogue for you to explore.
Information
Topic
Physical SciencesSubtopic
Optics & LightChapter 1
Raman effect
1.1Basics: the Raman virtual state
Raman effect was discovered by C. V. Raman in 1928[1-3]. It is an inelastic two-photon process, in which the scattering photon is first absorbed by the scatterer, for instance a molecule (in the followings, we will specify the scatterer to molecule without loss of generality). The photon-perturbed molecule is excited/disturbed and then relaxes and emits a secondary photon instantaneously. What excited/disturbed in the photon-perturbed molecule is its charges/electrons (from the ground state). During this process, the energy of the excited/disturbed charges may exchange with the molecular internal quanta, such as vibration through the so-called vibronic(vibration-electronic) coupling, so that the energy of the emitted photon may be different from the scattering photon. This energy difference is called the Raman shift. Raman shift is expressed in wavenumber (cm−1, wavenumber multiplied by the velocity of light, 3 × 1010cm/sec, gives the actual frequency). Raman shift gives the information of the molecular internal motion. For molecular vibration, Raman shift gives its frequency which is related to the molecular structure (geometry and atomic masses) and bond strengths. In fact, this information can also be obtained from other effects, such as infrared absorption. Hence, we know that the Raman shift is not the core information that we can obtain from the Raman effect.
The real core information lies in the Raman intensity which offers the dynamics of the photon-perturbed molecule. The photon-perturbed molecule, in general, may not be in its excited eigenstates. In such case, we call it the non-resonant the Raman process and the photon-perturbed molecule including its relaxation, the Raman excited virtual state, or simply the Raman virtual state. However, we have to stress that the Raman virtual state is a real physical entity, not just by our imagination. The word virtual should not be exaggerated to imply a physical meaning. The Raman virtual state is like a wave packet.
It is a common daily experience that in a pan full of water, there can be standing waves which are shaped by the pan. When a pebble is thrown into the pan, the water inside will be splashed above or even out of the pan in a random way. Similarly, when a molecule (the pan) absorbs a light quantum (the pebble), its electronic distribution (the water) will be disturbed. The disturbed (excited) electronic distribution in such an excitation is, in general, not stationary (the splashing water) or non-resonant and does not correspond to an eigenstate (the standing wave). We know that the eigenstates are governed by the molecular nuclei and can be accurately predicted by Schroedinger equation (just as that the patterns of the standing waves are defined by the pan boundary) while the non-stationary excitation including its relaxation, called virtual state, is not well defined by the nuclei and is hard to figure out though not impossible. This is the physical concept of the Raman virtual state. Just like that we cannot say that the splashing water is not a physical reality due to it is not a standing wave, we cannot say that the Raman virtual state is not a real physical entity due to that it is simply not an eigenstate or resonant one!
Back to the core issue: how to retrieve the information of the Raman virtual state from the Raman intensity? This is the central issue for the Raman study and the core topic we will cover in this book.
Before going to the issue of Raman intensity study, we will first introduce briefly the classical and quantum mechanical treatments of the Raman effect. If the readers are familiar with them, these sections may be skipped, except the Comments in Section 1.3.
Comments
If Raman excitation is to an eigenstate, we call the process resonant. In general, this is not the case, then the process is called non-resonant. In such case, no doubt that the electrons are disturbed and the molecule is in the so-called Raman virtual state. Hence, the virtual state is a physical reality, albeit the molecule is not in an eigenstate. Do not try to interpret the phenomenon from the vocabulary—virtual. It is only a word, a nomenclature for the phenomenon, not a deduction based on any physical considerations.
1.2The classical treatment
As a molecule is hit by the visible light (called the scattering light), the nuclear motion (vibration) will not be disturbed since its frequency is much less than that of the light. However, the electrons will be disturbed by the light. This disturbance of electrons by the external light is described by the electronic polarizability, which is a measure how easily the electrons can be affected by the light. As the electrons are more tightly bound to the nuclei and harder to be disturbed by the light, the electronic polarizability is smaller, otherwise, it is larger. The dimension of the electronic polarizability is volume. Its physical significance is the space occupied by the electrons. It is also proportional to the amount of electron numbers (charges). Since the electronic distribution of a molecule(or the molecular configuration/shape) is, in general, not spherical, the electronic polarizability is direction dependent, or a tensor. However, for the convenient elucidation of its physical significance, we will not go that far but simply consider it as a scalar in this book as we will pay more attention to the electronic polarizability of a bond. This will not affect the retrieval of the physical concepts of the topics concerned. For convenience, electronic polarizability is often shortened as polarizability.
Suppose the electric field of the scattering light with angular frequency ω is
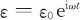
and the vibration is described by the normal mode (at least, in the low exci...
Table of contents
- Cover Page
- Title
- Copyright
- Preface
- Contents
- Chapter 1 Raman effect
- Chapter 2 Normal mode vibration
- Chapter 3 The elucidation of bond polarizabilities
- Chapter 4 The Raman virtual states
- Chapter 5 More applications
- Chapter 6 The extension to Raman optical activity
- Chapter 7 More applications on ROA
- Chapter 8 Intramolecular enantiomerism
- Chapter 9 A unified classical theory for ROA and VCD
- Appendix A One way to find out the bending coordinates that are more coupled to the stretching coordinates
- Appendix B References for the work on Raman and ROA intensities