
- 244 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
Statistical mechanics is concerned with defining the thermodynamic properties of a macroscopic sample in terms of the properties of the microscopic systems of which it is composed. The previous book Introduction to Statistical Mechanics provided
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Topic
Physical SciencesSubtopic
Mathematics GeneralChapter 1
Introduction
Problem 1.1 Prove from Eq. (1.1) that the integral in Eq. (1.3) is independent of path.
Solution to Problem 1.1
Equation (1.1) states the following integral around a closed cycle vanishes
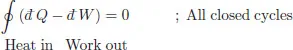
Pick two points on the cycle (A, B) with specified thermodynamic variables. Let C1 denote that part of the cycle running from A → B, and C2 that part of the cycle returning from B → A. Then the above states
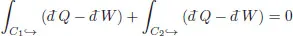
where the arrows indicate the direction during the cycle. Since both the heat flow and work are algebraic and change sign if the change occurs in the opposite direction, the integral changes sign if the trajectory is traversed in the opposite direction1
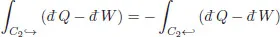
Hence the previous relation can be re-written as
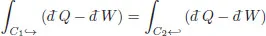
This states that the integral is the same whether we go from A to B along C1 or along C2 in the opposite direction. Since the cycle containing the points (A, B) is arbitrary, the integral
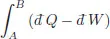
is independent of the path from A → B.
Problem 1.2 Start from either statement of the second law, and see how far you can get in verifying the statements leading to Eqs. (1.5) and (1.6); then compare with [Zemansky (1968)].
Solution to Problem 1.2
The two equivalent statements of the second law of thermodynamics are given at the start of section 1.1.2:
(1)Kelvin: It is impossible to construct an engine that, operating in a cycle, will produce no effect other that extraction of heat from a reservoir and performance of an equivalent amount of work.
(2)Clausius: It is impossible to construct a device that, operating in a cycle, will produce no effect other than the transfer of heat from a cooler to a hotter body.
We shall not provide a rigorous derivation of the consequences, but leave that to a basic course in thermodynamics.2 The key element is to establish that all reversible engines operating between two heat baths at distinct temperatures T1 > T2 have the same efficiency, equal to that of a Carnot engine, from which Eqs. (1.5)–(1.7) follow. The argument goes something like this. Consider two such engines operating between the two heat baths, and let the second operate in the reverse direction. Suppose the first absorbs heat Q1 at T1, produces work W, and expels heat Q2 at T2. Now put the heat Q2 and work W into the second engine. If it does not expel exactly the same heat Q1 into the bath at T1, then the combined engines constitute a device that can be arranged to violate the second statement of the second law.
Problem 1.3 (a) Why does each point on the dotted curve in Fig. 1.2 in the text correspond to a given T?3
(b) How could one carry out the Carnot cycles shown in Fig. 1.2 in the text in a continuous manner with each segment being covered only once?
(c) Why is it unnecessary for the construction of the entropy to actually traverse opposing segments of the adiabats in (b)?
(d) Show that the total heat input and total work output in (b) satisfy Q = W.
Solution to Problem 1.3
(a) A perfect gas obeys the equation of state PV = nRT (see, for example, Prob. 1.4). Thus if we specify (P, V), we also specify T;
(b) If we simply take the assembly around the outer segments of all the Carnot cycles, omitting all the common segments traversed in opposite directions, we move around the loop in a clockwise direction, in a continuous manner, with each segment being covered only once;
(c) The opposing segments along the adiabats in Fig. 1.2 in the text are traversed in a reversible manner, and along each of them the reversible heat flow vanishes, dQR = 0. Hence there is no change in entropy along those segments;
(d) Since the gas retu...
Table of contents
- Cover
- Halftitle
- Title Page
- Copyright
- Preface
- Contents
- 1. Introduction
- 2. The Microcanonical Ensemble
- 3. Applications of the Microcanonical Ensemble
- 4. The Canonical Ensemble
- 5. Applications of the Canonical Ensemble
- 6. The Grand Canonical Ensemble
- 7. Applications of the Grand Canonical Ensemble
- 8. Special Topics
- Appendix A Non-Equilibrium Statistical Mechanics
- Bibliography
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Introduction to Statistical Mechanics by John Dirk Walecka in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Mathematics General. We have over one million books available in our catalogue for you to explore.