![]()
Chapter 1
Ahmed Zewail: Ultra-Scientist and Citizen
Dudley Herschbach*
To my regret, I met Ahmed Zewail in person only a few times. We first met in Spring 1976, when he arrived at Caltech, newly appointed as an assistant professor. I was visiting that semester, on sabbatical leave from Harvard. Ahmed impressed me as very bright, cheerful, and witty, brimming with ideas and zest. About a decade later, although we had not met again, I was intrigued by his innovative papers. Particularly instructive was his exposition of the role of coherence in laser spectroscopy.1 Moreover, using molecular beams and ultrafast lasers, Ahmed had developed a pump-probe technique that mapped, in “real time,” trajectories of products from photoinduced reactions,2 exemplified by hν + ICN → I + CN. At first, the method was limited to such unimolecular reactions, initiated by photodissociation. Bimolecular reactions seemed inaccessible because means were lacking to define a time zero.
That limitation was soon overcome, as I learned at a workshop held at Jerusalem in November 1986, devoted to stereochemical aspects of chemical dynamics.3 Ahmed was not there, but Dick Bernstein had become a devoted apostle. A leading pioneer in molecular beam studies of chemical dynamics, Dick had, some years before, moved to the University of California at Los Angeles. Gleefully, he said he was “now a post-doc with Ahmed.” In that collaboration, Dick made a key contribution by advocating a technique then recently developed by Curt Wittig at the University of Southern California.4 It exploited the inherent mutual orientation of molecules in a weakly linked van der Waals adduct. The prototype case formed an XH … OCO adduct in a supersonic molecular beam. Then photolysis of the hydrogen halide initiated the bimolecular reaction, H + OCO → OH + CO, propelling the H atom into the nearby carbon dioxide. The collision was roughly collinear by virtue of the precursor geometry. Zewail’s team at Caltech used a picosecond laser pulse to photodissocate the HX, followed by a delayed picosecond laser probe of the OH product.5 Thereby, they were able to clock the formation and decay of the HOCO reaction complex. The key feature was that the first pulse established the zero of time for the bimolecular reaction, enabling a series of probe pulses to record the “real-time” evolution of the correlated products.
Soon Ahmed was able to make use of femtosecond lasers, initiated by Chuck Shank and colleagues at Bell Labs. That greatly enhanced the time resolution, launching the awesome saga of femtochemistry. The name was suggested by Dick Bernstein; he and Ahmed published in 1988 a fine overview of the evolution and prospects of femtochemistry.6 Ahmed baptized his prolific labs FEMTOLAND I, II, III and joyfully spoke of his “Femtosecond Dream” and “Femtocopia.” The overview sparkled with evangelical fervor, concluding with: “This happy marriage of ultrafast lasers and chemistry promises an exciting future for this field of real-time molecular reaction dynamics.”
As part of its centennial year, Caltech held a three-day symposium to celebrate Linus Pauling’s 90th birthday on February 28, 1991. A year before, Ahmed had been appointed to the newly endowed Linus Pauling Professorship for Chemical Physics. On that occasion, he mentioned with a smile that two days before he had become half as old as Pauling. Aptly, the symposium extolled both Linus and Ahmed. The lectures became a book, The Chemical Bond: Structure and Dynamics, edited by Ahmed.7 In his preface, he proclaimed that “as X-rays introduced the distance scale for molecular structure,” now the advent of femtochemistry has “introduced the time scale for the dynamics of the chemical act itself — the transition states between reagents and products.” Five chapters commemorated structure, two of them from lectures by Pauling, and the others by Alex Rich, Max Perutz, and Francis Crick. Four chapters dealt with dynamics: three from lectures by George Porter, John Polanyi, and me. The fourth chapter, not presented as a lecture, was the sparkling evangelical overview that Ahmed had published with Dick Bernstein,6 brought up to date. Dick had died in 1990; that chapter abides among his definitive legacy to chemical dynamics.8
Like many physical chemists of my vintage, I am among the many scientific grandchildren of Pauling. My Ph.D. mentor was one of his most distinguished scientific sons, E. Bright Wilson. I was particularly glad to take part in the Pauling symposium because, 33 years earlier, I first visited Caltech at the behest of Linus, when he was Chairman of the Chemistry Department. Back then I gave, for the first time, a talk about my fledgling plans and hopes for molecular beam studies of chemical reaction dynamics. My lecture at the Paulingfest sampled what grew out of those plans. Particularly, I emphasized how the role of electronegativity — a favorite theme of Linus — nicely explains some striking aspects of elementary reactions. Ahmed protested that I had made it too simplistic. Perhaps so, yet it seemed to capture the essence, emulating Linus. I pointed to a photo, shown here as Figure 1.1, which graced the dust jacket of The Chemical Bond book. It surely exemplified, in a simplistic way, his favorite concept of coherence!
A few months later, Linus, Ahmed, and I met again, to celebrate the award of the Pauling Medal to Rudolph A. Marcus. Ahmed gave a talk on the Br + I2 reaction, which proceeds via a “sticky” collision complex.9 His new FEMTOLAND II apparatus made it possible to determine the lifetime of the complex to be ∼50 ps. My talk, apropos of the 1991 presidential election campaign, was titled “How do electrons choose between candidate reaction products?” I passed out a ballot listing two candidates for each of the eight reactions. Molecular beam experiments had confirmed the predominant products, mostly “winners by a landslide.” But few of the voters chose more than 3/8 of the winners, although nearly all had Ph.D.s in chemistry. To my dismay, Ahmed and Rudolph each got only 2/8! Choices made on the basis of energetic or statistical considerations proved misleading. Again, Pauling-like heuristic reasoning predicted the winners, by simply taking account molecular orbital asymmetry arising from differences in electronegativity. Afterward, Ahmed sent me a brief letter, saying (diplomatically?) that his students “didn’t like molecular orbitals.”
Figure 1.1. Linus Pauling and Ahmed Zewail strolling on the Caltech campus with coherent strides.
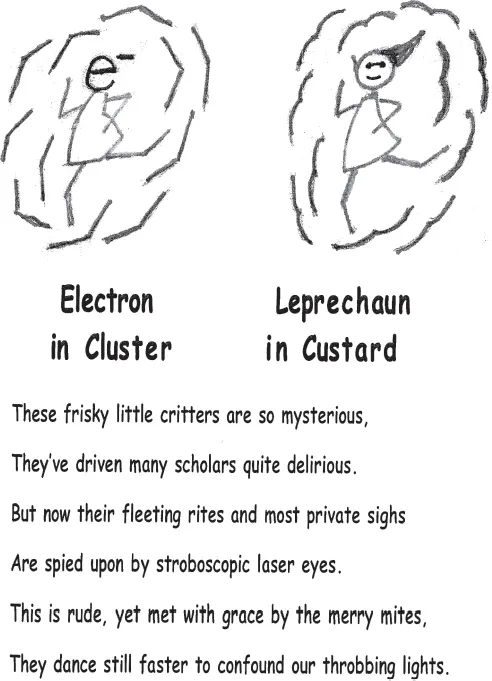
In November 1992, Ahmed visited Cambridge to deliver a joint Harvard–MIT physical chemistry seminar. As well as giving a scintillating talk on femtochemistry, he romped through visits with faculty, students, and postdocs, the day at Harvard before his talk and at MIT the day after. I had the opportunity to engage in a wide-ranging discussion with him for two hours in the morning and another four hours during and after dinner. Throughout, Ahmed was delightfully vivacious, both in describing his work and asking many searching questions. He was especially curious about a whimsical verse I had hung up near my desk (see Figure 1.2). I had composed it after a seminar given at Harvard in the early 1980s by Geraldine Kenney-Wallace. At Toronto, in 1974, she had set up an ultrafast laser that enabled her to elucidate the formation of solvated electrons. Back then, “ultrafast” meant picosecond. The format of my verse mimics that in a booklet of poems by Robert Williams Wood (1868–1955) titled How to Tell the Birds from the Flowers (1917). Wood was an outstanding physicist, celebrated for his work on spectroscopy, physical optics, infrared and ultraviolet photography, ultrasonics — and for his humorous enterprises. I had composed several poems using Wood’s format, which required coming up with two sketches with titles that are similar in looks and sound. Despite some effort, I had failed to concoct anything tractable for femtochemistry. Ahmed was gracious in accepting my apology. In turn, I noted that the words of the poem would apply to his femtochemistry, if the final line were changed to read: “They dance faster but cannot elude our throbbing lights.”
Figure 1.2. Verse inspired by solvated electrons tickled by a pulsed laser.
At dinner, among things prompted by Ahmed’s seminar, there came up a question about a quote he had shown on his last transparency slides (no PowerPoints yet!): “What good is a newborn baby?” It was attributed to Michael Faraday, responding to the query: “What will it be good for?” which greeted his discoveries in electromagnetism. As I was a life member of Friends of Benjamin Franklin (akin to Baker Street Regulars for Sherlock Holmes), I mentioned that the newborn baby response was made by Franklin in 1783, after observing in Paris the first flight of a hot-air balloon. The following week, Ahmed sent a letter asking for more information about the newborn baby quote. I obliged by sending copies of two scholarly articles by historians who had explored the question.10 I mention this minor episode because of Ahmed’s avid interest. Years later, he evidently had made a very thorough study of Franklin. Ahmed gave a splendid talk, “Franklin’s Vision,” at the Tercentenary celebration of Franklin’s birth held in 2006 at the American Philosophical Society in Philadelphia.11 In his office, Ahmed had Franklin’s bust, “as a daily reminder of what it means to be a scientist in service of society and a citizen of the world at large.”
Shortly after his visit, Ahmed sent a preprint of a theoretical paper, treating femtosecond dynamics on model potentials.12 It mapped out the motion of a wave packet and its dispersion on repulsive, attractive, and barrier-crossing potentials, obtaining estimates of reaction times and relating them to parameters of the dynamics. A key issue was the time scale over which wave packets remain coherent or localized. The analysis, replete with well-designed diagrams and easy-to-follow equations, demonstrated that, on the time scale of femtoseconds to picoseconds, the wave packet spreading is relatively insignificant. This paper, a contribution to a Festschrift celebrating my 60th birthday, included a fulsome dedication, concluding: “We wish him the best and expect the Herschbachian contributions to continue with every femtosecond full of joy!” My reply noted that 60 years contains 3 moles of femtoseconds.
In February 1993, I visited Caltech again to give the Pauling Lecture, hosted by Ahmed. On my arrival, he graciously thanked me for the congratulatory message I had sent him on the announcement of his Wolf Prize win. Then, laughing about the 1991 ballot, he brought up the role of molecular orbitals and electronegativity. He even praised the exposition of it in my chapter in The Chemical Bond book.7 Also, he had evoked a frontier molecular orbital in discussing the Br + I2 reaction.13 I had brought along the ballot. His colleague, Jack Roberts, had heard about it and asked to vote; he scored 6/8. Ahmed’s secretary, who never studied a chemistry course, also voted and scored 4/8, pleased that her score equaled the sum of Ahmed and Rudolph.
As well as discussing current experiments in our labs, Ahmed was eager to hear stories about Linus, especially some told to me by Bright Wilson.14 Ahmed, like me, had found the 1935 text by Pauling and Wilson, Introduction to Quantum Mechanics, unexcelled as an introduction (still in print!). Bright, as his Teaching Fellow, took notes on Pauling’s lectures. Bright recalled that Linus, when responding to a question, would often pretend that he had not considered it before. He would work out the mathematical solution on the blackboard, step-by-step, with commentary implying it was a fresh excursion. Bright knew that Linus had carefully prepared the derivation before class. Bright also told me that, although he did not remember which of them had written various parts of the book, those written by Linus were turned out at a steady pace, without any revision. Several of Ahmed’s co-authors on papers have testified that he too, in writing as in speaking, was remarkably adept and fluent.
Dinner the evening before my lecture was the last occasion during which I saw Linus. He told a story Ahmed enjoyed and had not heard before. Linus said that one day, just 15 minutes before his class, the phone rang. It was Ava Helen, his wife, so...