![]()
Chapter 1
Introduction
1.1.Process Intensification
1.1.1.History
The original thinking of “Process Intensification” (PI) was first introduced at Imperial Chemical Industries (ICI) by Colin Ramshaw and his colleagues during the 1970s (Reay et al., 2013), as a concept to reduce the capital cost by reducing the size of unit operations dramatically, with a size reduction factor that is expected to range from 2 to 1000 times (Stankiewicz and Moulijn, 2000).
The definition and content of PI for chemical engineering changed over the last decades. Initially, size reduction by heat/mass-transfer/mixing enhancement with HiGee technology was the main focus of PI exploitation. However, as Ramshaw and co-workers pointed out (Reay et al., 2013), the size reduction may not be the single objective. Besides, some early references related with the employment of HiGee forces in e.g. Podbielniak centrifugal contactor by rotating systems may even date back to 1930s, long before the PI expression appeared. Later, it became apparent that apart from cost reduction many other important advantages can also be achieved from PI. For instance, equipment miniaturization can increase the safety of chemical process without sacrificing product quality (Reay et al., 2013). Consequently, the interest of both chemical industry and academia in PI increased to a significant extent since the mid-1980s, and the arena for PI was broadened to fulfil the needs of modern chemical engineering development, including some key objectives:
•Reduce cost (CAPEX, variable cost, etc.).
•Reduce energy consumption and CO2 emission.
•Reduce raw material cost.
•Reduce environmental impact.
•Increase process flexibility and stability.
•Increase operating safety.
•Improve product quality.
Since PI is always associated with one or more of the aforementioned attractive benefits, Stankiewicz and Moulijn (2000) offered the following definition for PI:
Any chemical engineering development that leads to a substantially smaller, cleaner, and more energy efficient technology is process intensification.
However, the methodologies for achieving these benefits can be classified as PI, Process Systems Engineering or even classical process optimization, and such definition may bring difficulties to discriminate between them. A comparison of some basic features of these concepts, taken from one fundamental and comprehensive literature review by Van Gerven and Stankiewicz (2009) of PI, is given in Table 1.1, which is helpful to realize that PI differs essentially in character from the other methods.
Generally speaking, PI aims at drastically improving the performance of (or even realizing) a chemical engineering process by completely redesigning conventional operating units and unit operations. Therefore, the definition of PI in the European Roadmap for PI was described as: “Process Intensification provides radically innovative principles in process and equipment design which can benefit process and chain efficiency, capital and operating expenses, quality, wastes, process safety and more” (EFCE, 2007). Such vague expression still does not define the term PI in a rigorous way.
More recently, Lutzea et al. (2010) classified the three principles associated with PI: (1) integration of operations; (2) integration of functions; and (3) integration of phenomena, which could be more useful to determine whether an enhancement method is a PI or not. Additionally, there is still an on-going discussion about the definition of PI (Luo, 2013); different terms of its definition clearly show that the PI is a developing field of research, which is far away from a mature status. The development and implementation of PI has gained a worldwide R&D interest in recent years. For example, the European Commission funded the ‘F3 Factory’ project in 2009 (http://www.f3factory.com), which aims to deliver holistic process design methodology applying PI concepts, and a roadmap (10–40 years) for PI in Europe was published in 2008 by the Working Party on PI from the European Federation of Chemical Engineering (EFCE). For industrial applications, multiple European companies related with the chemical industry established the European Process Intensification Centre (EUROPIC) in 2008, which aims to accelerate the application of PI from an industry-driven platform. For academic research, there is an international scientific journal entitled Chemical Engineering and Processing with “Process Intensification” as subtitle specially focused on PI and related fields.
Table 1.1 A comparison between process systems engineering and PI. Reprinted with permission from Van Gerven and Stankiewicz (2009). Copyright (2016), American Chemical Society.
| Process systems engineering | PI |
| Aim | Multi-scale integration of existing and new concepts | Development of new concepts of processing methods and equipment |
| Focus | Model, software, numerical method | Experiment, phenomenon, interface |
| Interdisciplinarity | Modest (interface with applied mathematics and informatics, chemistry) | Strong (chemistry and catalysis, applied physics, mechanical engineering, materials science, electronics, etc.) |
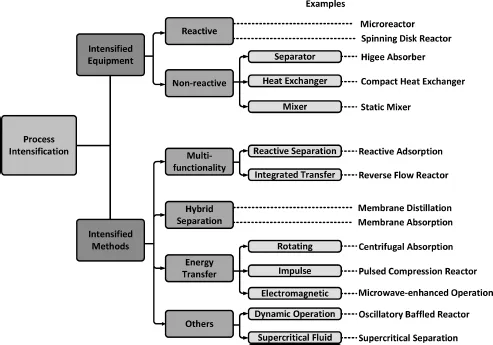
Figure 1.1 Classifications of PI equipment and methods. Adapted from Stankiewicz and Moulijn (2000).
1.1.2.Classification
As discussed in the above section, PI covers a wide range of R&D areas. As illustrated in Fig. 1.1, numerous PI technologies developed can be divided into two categories: Equipment and Methodology, which is similar as the concept of “hardware” and “software” for IT industry. The first classification, PI equipment, involves equipment for both reactive and non-reactive processes. For instance, reactive PI equipment such as structured reactors and spinning disc reactors are developing rapidly in recent years, while compact heat exchangers and static mixers are typical non-reactive PI equipment that has been employed in the chemical industry and other processes for a long time. On the other hand, one has a second category — PI Methodology, which can be classified into four different areas (Stankiewicz and Moulijn, 2002): integration of reaction and other unit operation(s) into multifunctional reactors, integration of separation and other unit operation(s) into hybrid separations, use of alternative forms of energy supply for chemical processes, and other techniques such as the use of new reaction–separation media, dynamic operating methods, etc.
As pointed out by Moulijn et al. (2013), the traditional classification (Fig. 1.1) focuses on the mesoscale (unit level) and macroscale (process level) PI. However, it might be even more advantageous to promote PI into smaller scales, since micro- (particle level) and nanoscale (molecule level) are the scales where the basic reaction, separation, mass and heat transfer take place and the improvement in these scales for PI can be more obvious. A good example is the development of multifunctional materials (Cunha et al., 2014). Comparing with multifunctional reactors (unit level), by combining a catalyst (reaction) and an adsorbent (separation) to form a “multifunctional reactor” at the particle level one is able to intensify mass and heat transfer at the microscale. In this book, literature related with the synthesis and implementation of such multifunctional materials for PI will be discussed (cf. Section 2.3.3).
1.2.Integration of Reaction and Separation Operations
1.2.1.Multifunctionality
Integration of functions, also known as functionality intensification, is one of the essential principles for PI (Lutzea et al., 2010), which can be illustrated through integration of conventional reaction with other functions into multifunctionality operation. The other functions can be mass, heat and momentum transport, or other reaction and separation unit operations (Agar, 1999). Among these integrated functions, the reactive separation process, which involves the integration of chemical reaction with other separation operation(s), is considered as the most widely applied functionality intensification method (multifunctional reactor) in the chemical industry (Sundmacher and Qi, 2010).
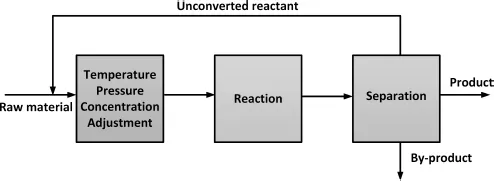
Most of the chemical reactions are limited by the equilibrium between reactants and products. As a result, it is necessary to introduce downstream separation processes to separate the equilibrium mixtures and to recycle the unconverted reactants, as shown in Fig. 1.2.
Figure 1.2 Standard process for a reversible reaction of which the conversion is limited by the thermodynamic equilibrium.
The number of separation steps is mainly dependent on the number of products produced, number of solvents used and reactants that have not been converted. The more separation steps used, the more operation procedures and energy input are required. In order to reduce the costs of separation processes, the concept of integration of reaction unit(s) and separation unit(s) into one device has been derived (Schmidt-Traub and Górak, 2006).
In this integrated unit, the product compound(s) is(are) continuously being separated from the reaction zone. In this way, a higher conversion of the reactant(s) can be achieved, and less separation steps will be required. The major characteristic of an integrated reaction and separation unit is the existence of at least two phases, a reaction phase and a transport phase (Sundmacher et al., 2005). Depending on the separation properties, on the reaction phase and transport phase, a set of reactive separation methodologies developed for multifunctional reactors can be found — cf. Fig. 1.3.
When an adsorbent is used, the process is called reactive adsorption and the reactor can be classified as adsorptive reactor, chromatographic reactor or simulated moving bed reactor (SMBR), depending on the phase and operating method used. On the other hand, a permselective membrane is used for a membrane reactor (MR). Adsorptive reactors (Chapter 2), MRs (Chapter 3) and SMBRs (Chapter 4) for PI will be described and exemplified in this boo...