
Perovskite Solar Cells: Principle, Materials And Devices
Principle, Materials and Devices
- 244 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Perovskite Solar Cells: Principle, Materials And Devices
Principle, Materials and Devices
About this book
-->
Energy and climate change are two of the most critical issues nowadays. These two topics are also correlated to each other. Fossil fuels are the main energy supplies that have been used in modern history since the industrial revolution. The impact of CO 2 emission has been a major concern for its effect on global warming and other consequences. In addition, fossil fuels are not unlimited. Due to the increasing demands for energy supplies, alternative renewable, sustainable, environmentally friendly energy resources are desirable.
Solar energy is an unlimited, clean, and renewable energy source, which can be considered to replace the energy supply of fossil fuel. The silicon solar cell is one of the dominant photovoltaic technologies currently, which converting sunlight directly into electric power with around 20% efficiency. This technique was been widely used in mainstream solar energy applications for decades, though the relatively energy-demanding production process remained with challenges to be resolved.
Recently, emerging photovoltaic technologies such as organometal halide hybrid perovskite solar cell has attracted tremendous attention due to their promising power conversion efficiencies (over 22%) and ease of fabrication. Their progress roadmap is unprecedented in photovoltaic history from the material development and efficiency advancement perspective. Beyond the rapid progress achieved in the last few years, it is expected that this novel technology would make an impact on the future solar cell market providing long-term stability and Pb content issues are addressed. These challenges rely on a better understanding of materials and device function principles. The scope of this book is to provide a collection on the recent investigations from fundamental process, materials development to device optimization for perovskite solar cells.
--> Contents:
- Additive-Assisted Controllable Growth of Perovskites (Yixin Zhao and Kai Zhu)
- Control of Film Morphology for High Performance Perovskite Solar Cells (Cheng-Min Tsai, Hau-Shiang Shiu, Hui-Ping Wu and Eric Wei-Guang Diau)
- Sensitization and Functions of Porous Titanium Dioxide Electrodes in Dye-Sensitized Solar Cells and Organolead Halide Perovskite Solar Cells (Seigo Ito)
- P-Type and Inorganic Hole Transporting Materials for Perovskite Solar Cells (Ming-Hsien Li, Yu-Hsien Chiang, Po-Shen Shen, Sean Sung-Yen Juang and Peter Chao-Yu Chen)
- Hole Conductor Free Organometal Halide Perovskite Solar Cells: Properties and Different Architectures (Sigalit Aharon and Lioz Etgar)
- Stability Issues of Inorganic/Organic Hybrid Lead Perovskite Solar Cells (Dan Li and Mingkui Wang)
- Time-Resolved Photoconductivity Measurements on Organometal Halide Perovskites (Eline M Hutter, Tom J Savenije and Carlito S Ponseca Jr)
-->
--> Readership: Graduate students and researchers in chemistry, materials science and photovoltaics. -->
Keywords:Perovskite Solar Cells;Hole Transporting Materials;Stability;THz SpectroscopyReview:0
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
| 1 | Additive-Assisted Controllable Growth of Perovskites |
| DSSC | dye-sensitized solar cell |
| EDX | energy-dispersive X-ray |
| FTO | fluorine-doped tin oxide |
| IPA | isopropanol |
| Jsc | short-circuit current density |
| MAI | methylammonium iodide |
| MAPbI3 | methylammonium lead iodide |
| PCE | power conversion efficiency |
| PSC | perovskite solar cells |
| PV | photovoltaic |
| SEM | scanning electron microscopy |
| TGA | thermogravimetric analysis |
| TGA-MS | thermogravimetric analysis-mass spectroscopy |
| Voc | open circuit voltage |
| XPS | X-ray photoelectron spectra |
| XRD | X-ray diffraction |
I. Introduction
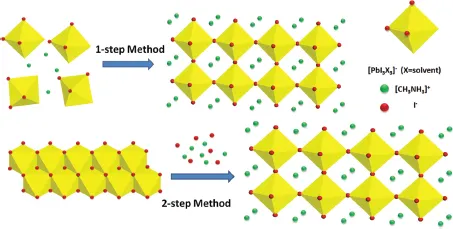
II. Cl-Based Additives
A.Role of MACl in One-Step Solution Processing
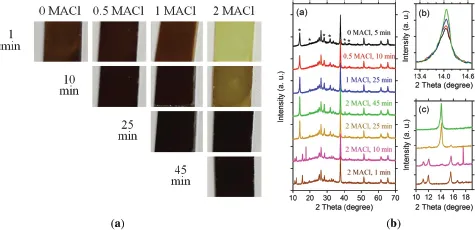
Table of contents
- Cover
- Halftitle
- Title
- Copyright
- Contents
- Preface
- 1 Additive-Assisted Controllable Growth of Perovskites
- 2 Control of Film Morphology for High-Performance Perovskite Solar Cells
- 3 Sensitization and Functions of Porous Titanium Dioxide Electrodes in Dye-Sensitized Solar Cells and Organolead Halide Perovskite Solar Cells
- 4 P-Type and Inorganic Hole Transporting Materials for Perovskite Solar Cells
- 5 Hole Conductor Free Organometal Halide Perovskite Solar Cells: Properties and Different Architectures
- 6 Stability Issues of Inorganic/Organic Hybrid Lead Perovskite Solar Cells
- 7 Time-Resolved Photoconductivity Measurements on Organometal Halide Perovskites
- Index