![]()
Chapter 1
OPTICALLY MODULATED THERANOSTIC NANOPARTICLES
Niksa Valim and Amit Joshi*
Department of Radiology, Baylor College of Medicine,
Houston, TX 77030, USA*[email protected] Abstract
Nanoparticles are rapidly advancing as drug carriers for directing therapies in a spatio-temporally controlled manner. By virtue of their size ranging from tens to hundreds of nanometers, nanoparticles can preferentially trap in tumors and aberrant vascular interstitium, and can be targeted to specific molecular pathologies. Theranostic nanoparticles is an umbrella term designating the carriers, which combine both the drug/therapy transport with an imaging contrast. With suitable targeting, theranostic nanoparticles can be used for molecular imaging, and potentially for monitoring therapy response.1 To enhance therapy specificity to the disease site and reduce off-target effects, external and direct spatial-temporal control on therapy delivery is desirable.2 Multiple approaches for modulating theranostic nanoparticles have been reported, viz. magnetic fields, sonic energy, and optical radiation. Optical modulation in the range of visible and near infrared (NIR) wavelengths is a valuable tool for theranostic nanoparticle modulation because of its non-ionizing and safe nature, tissue penetration of multiple centimeters, easy multiplexing by including multiple interrogation wavelengths/multiple fluorophores, low-cost, and advances in miniaturization of point of care optical illumination/detection devices. Optical stimulation is routinely used for noninvasive visualization of exogenous fluorescent markers, fluorescent proteins, or for triggering the delivery of drugs in humans and in laboratory animals.3 In this chapter, recently reported optically modulated theranostic nanoparticle platforms are described with a brief discussion on their biomedical applications and potential of translation to clinic. The nanoparticle platforms are organized according to the synthesis materials, with focus restricted to platforms exhibiting external optical control on diagnostic/therapeutic triggering.
1.1 Polymeric nanoparticles
Polymeric nanoparticles composed of large chain organic molecules and synthetic/natural polymers comprise the largest and best-studied class of theranostic nanocarrier systems because of their following unique characteristics; (i) they have controllable physiochemical properties such as particle size, surface charge, and degradation rate, (ii) they can transfer different types of therapeutic or imaging agents, incorporating their unique hydrophilicity or hydrophobility profiles within the controllable nanoparticle architecture, and (iii) their surfaces can be modified for binding to targeting moieties via relatively simple and reproducible chemistry techniques.4–6
Polymeric nanoparticles encapsulating photosensitizers were demonstrated as one of the first truly optically modulated theranostic nano-platforms.7,8 Their imaging and therapeutic applications are respectively due to strong fluorescence emission and generation of cytotoxic singlet oxygen from the photosensitizers under irradiation of light with appropriate wavelength. While photosensitizers such as porphyrin derivatives have been used for imaging and therapy for at least a decade,9,10 some of their intrinsic characteristics limit their widespread application. First, most photosensitizers are barely soluble in physiological conditions. Second, they may cause severe phototoxicity and photosensitivity in patients’ skin and eyes when exposed to the sun up to several months after treatment. Therefore, photosensitizers require suitable delivery carriers to modulate their action in space and time, especially for their intravenous administration. Multiple polymeric nanoparticles have emerged for this purpose to date.
Weissleder and co-workers reported the production of poly nanoparticles (lactic-co-glycolic acid) that encapsulate meso-tetraphenylporpholactol photosensitizer as a theranostic nano-platform.7 The encapsulation of the photosensitizer stabilized them for systematic administration. Upon cellular internalization, the photosensitizer is released and the irradiation by visible light to the target area activated it for fluorescence emission and generation of singlet oxygen. Nanoparticles induced significant cytotoxicity to the cancer cells both in vitro and in vivo. Further advantage of these nanoparticles was their bio-degradability.
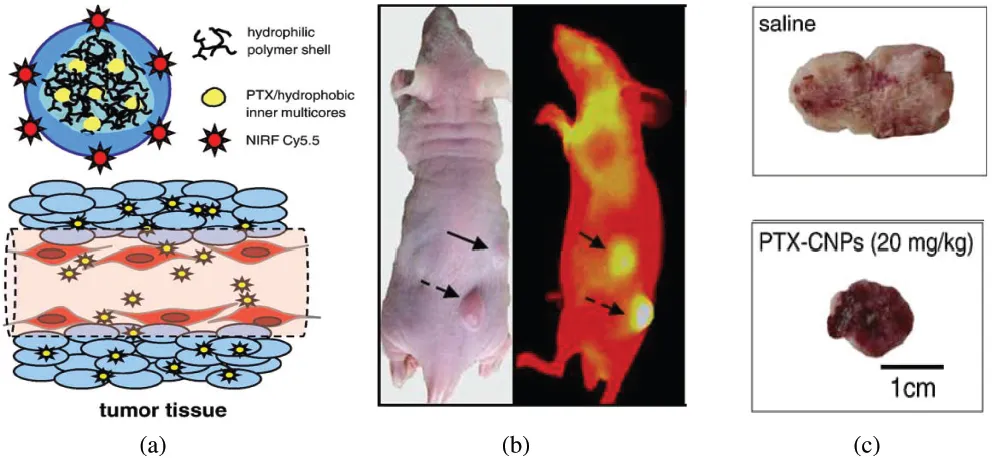
Kwon and co-workers reported polymeric self-assembled nanoparticles based on hydrophobic biocompatible/biodegradable glycol chitosan (HGC).11,12 These particles were shown to circulate through the blood stream for up to three days and preferentially accumulated in tumors via enhanced permeability and retention (EPR) effect. These particles were loaded with paclitaxel for cancer chemotherapy. Additionally, the HGC nanoparticles were labeled with Cy5.5, which is a near infrared fluorescent dye, for imaging. These polymeric nanoparticles were used for simultaneous visualization and treatment in SCC7 tumors in vivo. Figure 1.1 shows the schematic diagram of the polymeric nanoparticles along with an example of excised tumor treated with saline and paclitaxel-loaded HGC nanoparticles (PTX-CNP).
Figure 1.1 (a) Schematic diagram of chitosan-based nanoparticles, (b) in vivo images of SCC7 tumor. The black arrow indicates intravenous injection of pactitaxel loaded-HGC nanoparticles labeled with Cy5.5, and (c) representative images of excised tumors treated with Saline and PTX-CNP for 18 days (Reproduced with permission from Ref. 11, copyright © Elsevier, 2010.)
Chances of clinical translation of polymeric nanoparticles encapsulating photosensitizers are high, as photosensitizers on their own have progressed to initial clinical trials for esophageal and skin cancer.13,14 Coupled with favorable bio-degradation and safety profiles of carrier polymeric nanoparticles, we may potentially see clinical applications in the next decade.
1.2 Carbon nanotubes
Carbon nanotubes (CNTs) have unique physical and chemical properties and have been widely explored in the fields of drug delivery, biological imaging, and thermal ablation.15 CNTs are allotropes of carbon with cylindrical nanostructures. These cylindrical carbon molecules are categorized as single-walled nanotubes (SWNTs) and multi-walled nanotubes (MWNTs). SWNTs are made up of a single rolled up layer of graphene, while MWNTs consist of multiple tubes of graphene sheets. CNTs are able to load targeting ligands and chemotherapy agents.16–18
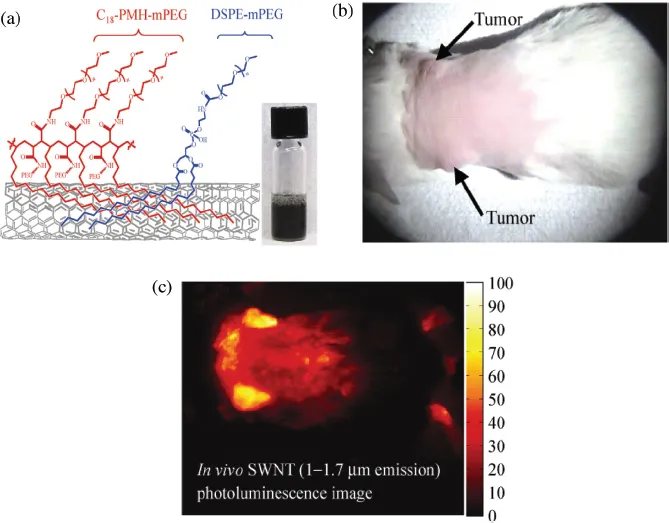
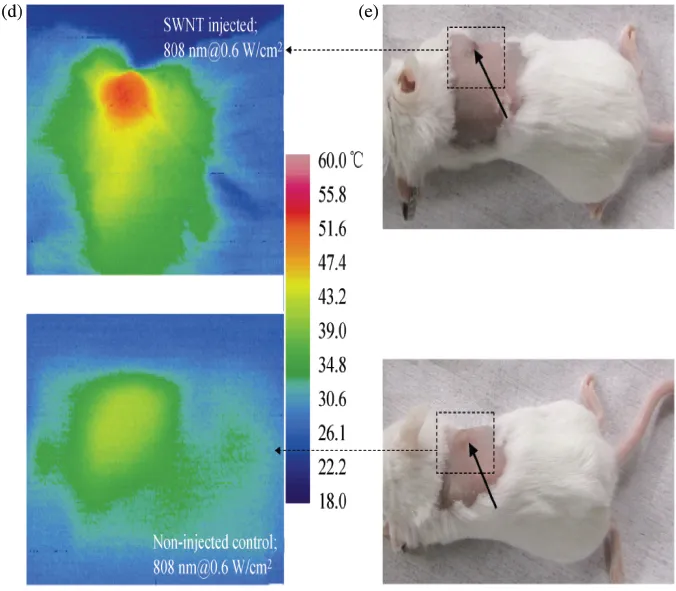
Dai and co-workers have studied the exposure of SWNTs to laser light excitation in the biological optical window, i.e. in the range of 700 nm to 900 nm wavelengths. This excitation wavelengths were could induce thermal ablation of cancer cells both in vitro as well as in vivo.19 They functionalized SWNTs by PEGylated phospholipids with non-toxic and long-circulating properties. In order to demonstrate in vivo measurements, 4T1 murine breast tumor cells were injected subcutaneously in 20 BALB/c mice right shoulders to obtain tumor-bearing mice. Five days after the inoculation, 10 mice were injected with 200 μL of a 2 μmol/L solution of SWNTs through intravenous tail injection. Figure 1.2(a) shows the schematic diagram of SWNTs with the coatings. These nanotubes were coated with a 50% DSPE-mPEG/50% C18-PMH-mPEG. The fluorescence of nanotubes was monitored in vivo for 48 hours using a 2DInGaAs detector. The other 10 mice remained untreated as controls. Three days after injection, all mice were irradiated with an 808 nm NIR laser for 5 minutes at 0.6 W/cm2 with a laser spot size of 4.4 cm2. The temperature was monitored continuously with thermal imaging. The images showed that the tumors on non-SWNT injected mice heated up very slowly and reached an average temperature of 43°C. However, the tumors of SWNTs injected mice heated up very fast and after 5 minutes of irradiation the temperature reached up to 52.9°C. All tumors in the control NIR radiation group without SWNTs injection did not show any sign of treatment, and all mice were considered as “non-survival” by the 36th day after thermal therapy. However, the tumors shrank in all SWNT injected and laser treated mice on average about two weeks after the treatment. All 10 mice in this group were considered survivors by ~6 months post-treatment without any tumor relapse.
Figures 1.2(b) and 1.2(c) depict optical imaging and SWNTs photo-luminescence imaging of BALB/c mice with breast tumors on both shoulders.Figure 2(d) depicts thermal imaging of an SWNT-injected mouse taken 4.5 minutes into NIR laser irradiation (top image) and a control mouse with the same irradiation (bottom image). Figure 1.2(e) shows the optical image of the BALB/c mouse injected with SWNTs (top image) and non-injected mouse (bottom image) immediately before NIR laser irradiation.
Figure 1.2 (a) Schematic diagram of SWNTs coated with DSPE-mPEG and C18-PMH-mPEG, (b) Optical image of a BALB/c mouse with two 4T1 tumors (indicated by arrows), (c) NIR photo-luminesce image taken 48 hours post-injection, (d) thermal images of a tumor-bearing mouse injected with solubilized SWNTs (top image) and non-injected mice (bottom image), and (e) optical image of the BALB/m mouse immediately before laser treatment with SWNT injection (top image) and non-injected SWNT (bottom image). (Reproduced with permission from Ref. 19, copyright © Tsinghua University Press and Springer-Verlag Berlin Heidelberg, 2010.)
In addition to tumor cell treatment with SWNTs, Dai and co-workers have reported photothermal ablation of vascular inflammation using these nanoparticles.15 Aqueous SWNT suspension was functionalized with Cy5.5 fluorescent dye and molecular and cellular imaging was possible with fluorescence imaging via NIR light irradiation. They have shown that SWNTs were taken up by macrophages both in cell culture and in mice carotid artery ligation. Cy5.5-SWNTs were injected intravenously via the tail vein, and fluorescence imaging was taken 24 and 48 hours ...