
A-Level Chemistry's Best Kept Secrets!
What Top Students Know That You Don't
- 224 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
A-Level Chemistry's Best Kept Secrets!
What Top Students Know That You Don't
About this book
-->
A-Level Chemistry's Best Kept Secrets! aims to inter-link and integrate different concepts and topics and it is written with the latest syllabus in mind. With effect from 2017, there would be a change in the A-level syllabus and the ability to cross link topics is integral to acing chemistry. Many guidebooks are of the expository and non-refutable type in which facts are presented rather than explained. In this book, novel and more efficient ways of looking at problems in chemistry are proposed to ensure good understanding of the subject.
-->
Request Inspection Copy
--> Contents:
- General and Physical Chemistry:
- Atoms, Molecules and Stoichiometry
- Atomic Structure
- Chemical Bonding
- The Gaseous State
- Chemical Energetics
- Electrochemistry
- Equilibria Part 1: Chemical Equilibria
- Equilibria Part 2: Ionic Equilibria
- Reaction Kinetics
- Inorganic Chemistry:
- Chemical Periodicity
- Group II
- Group VII
- Introduction to Transition Elements
- Organic Chemistry:
- Introduction — Structure and Bonding
- Introduction — Isomerism
- Hydrocarbons Part 1: Alkanes
- Hydrocarbons Part 2: Alkenes
- Hydrocarbons Part 3: Arenes
- Halogen Derivatives
- Hydroxy Compounds
- Carbonyl Compounds
- Carboxylic Acids and Their Derivatives
- Nitrogen Compounds: Amines, Amides and Amino Acids
-->
--> Readership: Students taking Singapore-Cambridge GCE A-level examinations, chemistry teachers, chemistry enthusiasts, chemistry major undergraduates. -->
Keywords:A Level Chemistry;H1;H2;H3;CIE;GCE;IBReview: Key Features:
- With effect from 2017, there would be a change in the A-level syllabus and the ability to cross link topics is integral to acing chemistry. Throughout the book, much guidance is given "on the spot" and an attempt has been made to interlink different topics and concepts as required by the new syllabus
- Novel and more efficient ways of looking at problems are proposed. Such methods and techniques have been refined through my years of teaching experience with international students
- Extra emphasis based on fundamental understanding has been placed on the Organic Chemistry section to enable the students to recall the reagents and conditions easily
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
SECTION 1
GENERAL AND PHYSICAL CHEMISTRY
SECTION 1.1
ATOMS, MOLECULES AND STOICHIOMETRY
Mole Equations
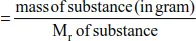
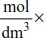
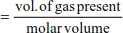
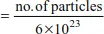
Example 1
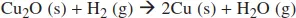
Solution
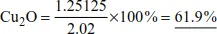
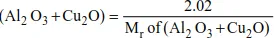
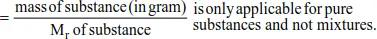
Example 2

Solution
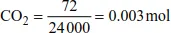
| no. of moles of C | = no. of moles of CO2 (from the formula, CO2, and the equation, C + O2 → CO2) |
| = 0.003 mol |

Example 3
Solution
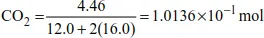
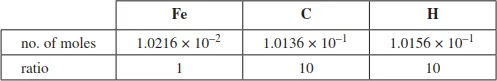
| no. of moles of C | = no. of moles of CO2 (from the stoichiometry of the reaction) |
| = 1.0136 × 10−1 mol |
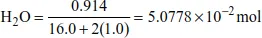
| For a given formula of H2O, no. of moles of H | = no. of moles of H2O × 2 (critical step) |
| = 5.0778 × 10−2 × 2 | |
| = 1.0156 × 10−1 mol |
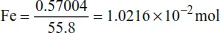
Calculations Involving Gases
Molar volume of gas at r.t.p (25oC and 1 atmosphere) is 24.0 dm3.
Table of contents
- Cover page
- Title page
- Copyright
- About the Authors
- Preface
- Acknowledgments
- Section 1 General and Physical Chemistry
- Section 2 Inorganic Chemistry
- Section 3 Organic Chemistry
- Dr Ernest Wong’s Testimonial
- Index