![]()
Chapter 1
Introduction
A ceramic material is an inorganic and non-metallic solid material that is composed of metal, non-metal and metalloid atoms bound by covalent or ionic bonds. The ceramic materials can range from crystalline and semi-crystalline to amorphous (glass) depending on the ordering of the structure. Generally, the ceramic materials have a high melting temperature, poor thermal and electrical conductivities, high hardness and low ductility. Also, the ceramic materials are lighter (less dense) than metals and heavier than polymers. They normally possess high thermal resistance, but low resistance for heat shock. Because most elements and types of bonding, and all range of crystallinity can comprise the ceramics materials, a lot of materials such as oxide, nitride and carbide materials are included in this category. Accordingly, the ceramic materials have been employed in a wide range of applications, such as in the manufacturing of bricks, tiles, dishes and vases.
The ceramic materials have been widely used in our daily lives since the ancient times. The earliest ceramics made by human are thought to be pottery objects. It was made of clay, either alone or mixed with other materials like sand (silica), followed by hardening and sintering by fire. Later, glazes were discovered and developed. They are normally glassy and amorphous ceramics with low melting point, and they can decrease the porosity of crystalline ceramic surface once they are coated on them. Because of the glazes, a smooth and colored ceramic surface can be obtained, and so a wide range of ceramic art was developed. Ceramics now include domestic, industrial and building products as well as ceramic art. In the 20th century, new ceramics materials such as semiconductors appeared in advanced ceramic engineering [1]. Particularly, developing ceramics parts for gas turbine engines are expected to reduce the volume and weight of the cooling system and even limit operating temperatures due to the high thermal stability of the ceramic materials. Also, bioceramics, such as those used in dental implants and synthetic bones, have played an important role in the medical field. Other examples of bioceramics are pacemakers, kidney dialysis machines and respirators. Calcium phosphate-based ceramics have been used for bone substitution. These ceramics have a similar chemical composition and structure as the bones. In dental implants, a polymer–ceramic composite is used to fill the pores of ceramics. The high biocompatibility is strongly required for the bioceramics.
The properties of ceramic materials are a direct result of its crystal structure and chemical composition. In most ceramic materials, ions cannot migrate because all ions must form a rigid skeleton structure. However, in some ceramics, only one ion can move with a low energy barrier and as a result, these ceramics possess high ionic conductivities comparable to those of molten salts and liquid electrolytes. Such ceramics are called “ion conductive ceramics.” The ion conductive ceramics have gained much attention for a wide variety of electrochemical applications, ranging from power generation (e.g. solid oxide fuel cell, SOFC)and energy storage (e.g. battery and capacitor) to atomic switches [2], and intensively researched to improve their properties, especially ionic conductivity, and to clarify the origin of their high ionic conductivities. The ion conductive ceramics are also called “solid electrolytes” when they are applied in electrochemical devices. In particular, the application of ion conductive ceramics to energy storage devices has been prompted in many groups.
Nowadays, we are facing global warming problem due to emission of CO2 as a result of burning fossil fuels like gasoline. In order to build a sustainable society and use renewable energy, usage of natural energy sources such as wind and solar powers has been researched. However, the intermittent nature of natural energy sources precludes it from being a stable energy supply. Therefore, the development of energy storage devices is key to enable the usage of natural energy.
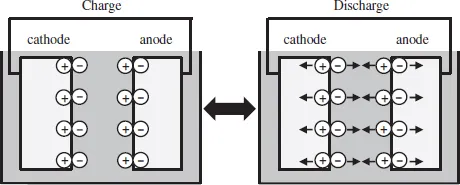
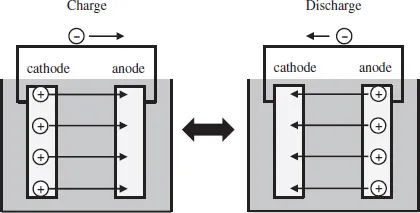
There are two kinds of electric energy storage devices, i.e. capacitors and batteries. The difference between these two devices is in the storage mechanism of electric charge. The capacitors store electric charges in the vicinity of the surface of the electrode (electrochemical double layer) (Fig. 1.1), while the batteries store the electric charges inside the electrode by Faradic reactions (Fig. 1.2). Therefore, the battery usually has higher capacity than the capacitors because the whole electrode can store the electric charges. On the contrary, the capacitors can charge and discharge faster than the batteries because they are not hindered by the kinetics of the Faradic reaction. The capacity of a capacitor is strongly influenced by the surface of the electrodes where electric charges are stored. Active carbon, which possesses a high surface area, has been used for the electrode material of a capacitor for this reason. However, electrode materials of batteries depend on battery reaction. For example, different electrode materials are used in Ni-hydrogen and lead-acid batteries.
Fig. 1.1Structure of a capacitor.
Fig. 1.2Structure of battery.
Some Faradic reactions are very fast. The storage devices using the fast Faradic reactions are also battery, but they behave like capacitors. Such capacitors are called pseudo-capacitors. In the pseudo-capacitors, the electric charges are thought to be stored inside the electrode, but they are probably stored only in the vicinity of the surface of the electrodes.
Electrolytes are put between cathode and anode to avoid short circuit. Normally, liquid electrolytes have been used in batteries. The liquid electrolyte sometimes causes serious safety issues like electrolyte leakage and evaporation. Especially, energy storage from natural energy resources requires a large-scale battery which contains more electrolytes, and thus the electrolyte problems also become more serious. Therefore, the development of solid electrolytes (ion conductive ceramics) has been intensively researched.
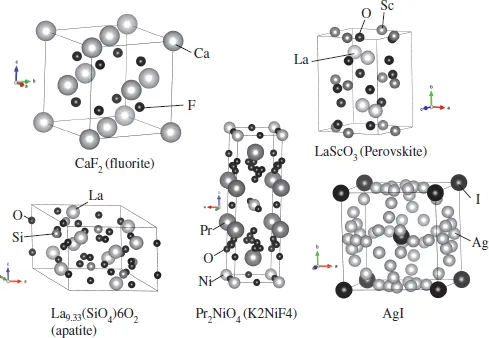
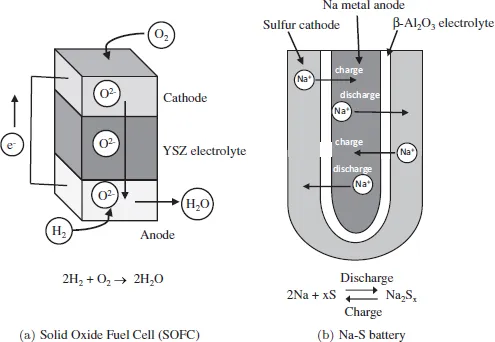
Among the innumerable researches on the conductive ceramics, many ion species such as O2– [3], Li+ [4–6], Ag+ [7], Na+ [8], Cu2+ [9], Mg2+ [10], Al3+ [11], Sn4+ [12], H+ [13] and so on have been found to migrate in ceramics depending on the structure of the ceramics and the temperature. Structures of fluorite-, perovskite-, apatite-, K2NiF4-type and AgI are well known as the ion conductive ceramics [14] (Fig. 1.3). AgI and Rb4Cu16I7Cl13 were thought to be suitable solid electrolytes for electrochemical devices due to their high ionic conductivity; however, their cost and difficulty in preparation have restricted their applications [15]. Alternatively, O2–, Na+ and Li+ conductive ceramics have been given more attention for application in electrochemical devices due to their high ion conductivities, low cost of production and wide electrochemical windows. The O2– conductive ceramics such as Yttria-stabilized zirconia (YSZ) can be used as a solid electrolyte in SOFC, which is expected to be employed in co-generation system and fuel-cell vehicles. In the SOFC, oxygen is supplied to the cathode and oxygen ion moves to the anode through the solid electrolyte. The oxygen ions produce water after reacting with hydrogen at the anode, as shown in Fig. 1.4(a). Usually, (La, Sr)MnO3 or LaSr(Co, Fe)O3 and Ni–YSZ cermet are used at the cathode and anode, respectively. This system can produce electricity with emission of only H2O, not CO2, and hence can be expected to be a clean power generation system. On the other hand, Na+ conductive ceramics such as β-Al2O3 solid electrolyte were successfully developed for the Na–S battery, which has been already commercialized. Na metal and sulfur are used as anode and cathode, respectively (Fig. 1.4(b)). Na ions migrate from the anode to the cathode through the β-Al2O3 solid electrolyte during discharging. At the cathode, sulfur reacts with Na and forms a Na–S alloy. This system possesses a high energy density — about three times higher than that delivered by a conventional lead-acid battery — long life and low cost(all components are abundant). This system is being used in factories and power plants for load-levelling and emergency power supply. Both systems do not contain any liquid components. Therefore, some issues like electrolyte leakage and evaporation, which are sometimes caused by the liquid electrolyte in the conventional commercial batteries such as nickel-hydrogen, Li and lead-acid batteries, never occur in these systems.
Fig. 1.3Structure of solid electrolytes.
Fig. 1.4Structure of (a) SOFC and (b) Na–S battery.
The currently available commercial Li batteries contain flammable organic liquid electrolyte. The flammability of the electrolyte is always a concern with regard to safety of the Li batteries as well as electrolyte leakage and evaporation, particularly in the large-scale development of Li batteries for electric vehicle (EV) and hybrid vehicles (HV) and for stationary energy storage. Li-ion conductive ceramics have b...