
Spectroscopic Identification of Organic Molecules
- 204 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Spectroscopic Identification of Organic Molecules
About this book
Researchers in the fields of organic synthesis, pharmaceutical research as well as cosmetic and agrochemicals industries need to confirm the structures of products they obtain. This was previously a time-consuming a process that took up much time. Spectroscopic methods however, made it easier, and initially R and UV helped chemists conclude structures one way or another. Initially 1D NMR, 2D NMR and sophisticated NMR measurements like COESY and NOESY were of great assistance. We demonstrate principles to conclude structures of our simple molecules, mainly heterocycles of interest for researchers in fields indicated above.
However, it is insufficient to only understand the principles, and one should also master problem solving and thinking. We demonstrate simple problems like the utility of coupling constants, NOE, COESY and NOESY and show how firm conclusions are obtained in real life. Most NMR books usually demonstrate these principles utilizing a pit sophisticated examples. The methodology suggested by us is simpler and quite useful for researchers in heterocyclic chemistry where combination of proton and carbon NMR should be dealt together.
Our research results previously been used intensively (Cf. citations in Google Scholar) and still draw attention. Students in the fields indicated will find this book of value to sign the spectra of the molecules they synthesize. Researchers in the field of heterocyclic chemistry as well as instructors in the field of structure proof utilizing spectroscopic identification will also find this book of interest.
Contents:
- Solving Research Problems Prior to Use of Spectroscopy
- Utility of X-Ray Diffraction
- Classical NMR as a Tool for Elucidating Structures
- Diastereotopic Protons
- Science Policy: Personal Experience
Readership: Graduate students and researchers on heterocyclic chemistry.
Key Features:
- Scientific Departments particularly in Science and Pharmacy institutes
- Research & development departments all over the world
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
| 1 | Solving Research Problems Prior to Use of Spectroscopy |
1.1.General Considerations
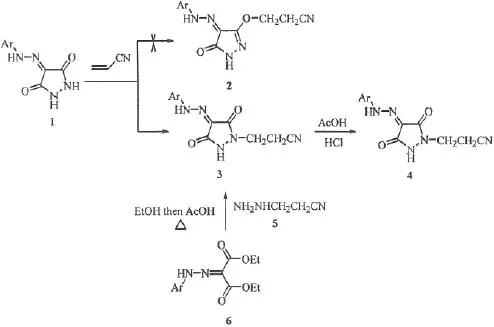
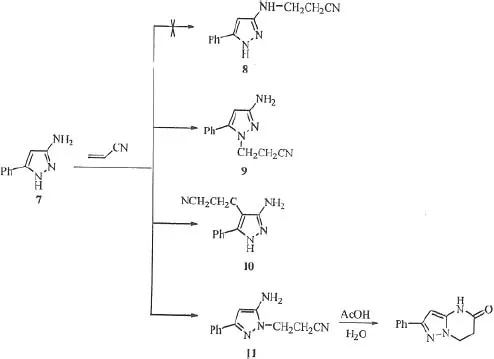
References
| 2 | Utility of X-Ray Diffraction |
2.1.General Consideration
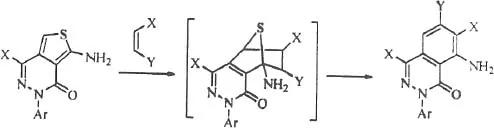
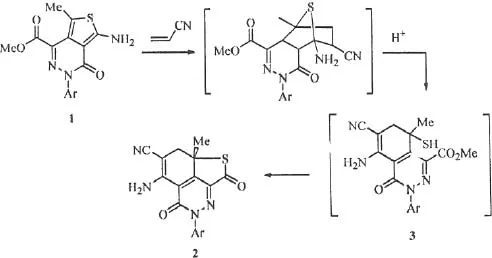
Table of contents
- Cover
- Halftitle
- Title
- Copyright
- Acknowledgments
- Contents
- Introduction
- Chapter 1 Solving Research Problems Prior to Use of Spectroscopy
- Chapter 2 Utility of X-Ray Diffraction
- Chapter 3 Classical NMR as a Tool for Elucidating Structures
- Chapter 4 Diastereotopic Protons
- Chapter 5 Science Policy: Personal Experience
- Index
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app