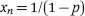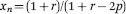![]()
CHAPTER 1
Microstereolithography
SHANGTING YOU1, KATHLEEN MILLER1 AND SHAOCHEN CHEN*
Department of NanoEngineering, University of California, San Diego, La Jolla, CA 92093, USA
*Email:
[email protected]1.1 Introduction
Fabrication is a critical process in making materials into functional parts and devices. Traditional fabrication technologies such as machining and molding are commonly used in macro-scale three-dimensional (3D) fabrication. However, they are not adequate for microscale fabrication. Modern micro- and nanoscale fabrication technologies such as photolithography, soft lithography, electron beam lithography, focused ion beam lithography, dip-pen lithography, and atomic layer deposition, are often 2-dimentional (2D) in nature for thin film and surface patterning.
3D printing, as an additive free-form 3D fabrication technology, has achieved great commercial success in the past decade, because of its low cost, simplicity, and versatility. Microstereolithography is a light-assisted 3D free-form fabrication technology. This technology utilizes photosensitive materials, which can solidify upon ultraviolet (UV) or short wavelength visible light exposure. By spatially controlling the exposure dose, the desired 3D structure can be fabricated. This technology evolves in two directions. One, scanning-based microstereolithography, which provides the extremely fine resolution (sub-micron scale) but slow fabrication speed (e.g., hours). Two, projection-based microstereolithography, which provides both fine resolution (micron scale) and fast fabrication speed (e.g., seconds to minutes).
Microstereolithography is a powerful tool for biofabrication.1 It has successfully demonstrated its capability of fabricating a wide range of biomaterials such as hydrogels, proteins, and cell-laden materials. Both synthetic and natural materials can be used to print, each having different advantages for stability, mechanical properties, cytocompatibility, and printability. Material decisions must come from the eventual application for the printed structure, as even the choice of photoinitiator can greatly impact the print.2,3
This chapter covers basic physical and chemical mechanisms in photopolymerization, materials, devices, and systems of microstereolithography. The photopolymerization mechanism is detailed in Section 2. Materials for microstereolithography are discussed in Section 3. Scanning-based microstereolithography, including single-photon polymerization microstereolithography, and two-photon polymerization nano-stereolithography, is detailed in Section 4. Projection-based microstereolithography, including liquid–air interface polymerization and liquid–substrate interface polymerization, is discussed in Section 5.
1.2 Photopolymerization
In this section, we will focus on photopolymerization, a technique most often used to crosslink liquid state monomers or oligomers into solid state long-chain polymers. Photopolymerization uses free radicals to initiate and crosslink strands within a monomer solution to form a solid hydrogel. When paired with microstereolithography techniques, various complex structures can be fabricated.4,5
1.2.1 Step-growth Polymerization
Two of the main types of polymerization observed in hydrogel scaffolds are step-growth and free-radical polymerization. Although photopoylmerization methods use free radicals to polymerize structures, the kinetics of step-growth polymerization can describe some of the more unique polymerizations, and thus it is important to cover.6,7 Step-growth polymerization occurs when polymer chains grow in a stepwise fashion either by condensation reactions, in which water is removed, or when reactive end groups interact. When considering simple linear chain reactions, the mechanisms and rates of all polymerization steps can be assumed as equal. Moreover, the Carothers equation (eqn (1.1)) defines the level of completion of the step-growth polymerization
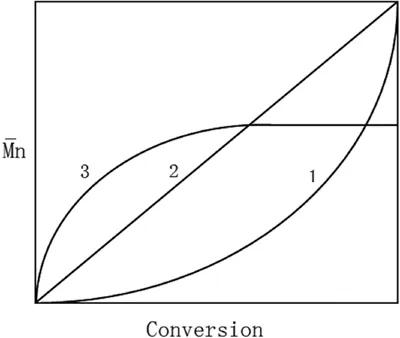
where xn is the average chain length, and p is the conversion rate of the monomers into polymers. This is an incredibly useful equation for predicting how long the reaction needs to proceed to obtain the correct molecular weight.8 As one can see, this defines an exponential relationship (Figure 1.1).9 As one might imagine, high molecular weights become increasingly more difficult to achieve for three reasons: (1) the frequency of reactive end groups meeting decreases; (2) the frequency of side reaction interference increases; and (3) when two or more monomer types are used in the reaction, it is difficult to ensure that the starting material concentrations are equal. This third point is an issue for users creating copolymer hydrogels, such as those consisting of monomer A and monomer B, where A-A does not react, nor B-B, but only A-B. To stop the reaction at a lower molecule weight, the user has a few options. One, the reaction can be rapidly cooled at the correct time point to slow down the polymerization rate as many step reactions have high activation energies. Two, a monofunctional material can be added to “cap” the end of the polymer, preventing it from further reactions. Lastly, if making a copolymer, a stoichiometric imbalance of the starting materials can be used. For example, if more A groups are used than B, eventually the polymer will have two A end groups on a chain and the B monomer will be completely consumed preventing further reactions with that polymer. The Carothers equation can be expanded to define this scenario, shown in eqn (1.2),
where xn is the average chain length, p is the conversion rate of the monomers into polymers, and r is the ratio of monomer A to monomer B (NA/NB).8
Figure 1.1 Number-average molecular weight (M̄n) vs. monomer conversion curves for step growth polymerization (1); living polymerization (2); and free-radical polymerization (3).
Reproduced from ref. 9 with permission from The Royal Society of Chemistry.
1.2.2 Free-radical Polymerization
In free-radical polymerization, a free radical interacts with reactive end groups to form a polymer. The basic structure of the active group is CH2CR1R2, as the pi-bond in the carbon–carbon double bond allows it to be rearranged when exposed to a free radical. From here forward this will be referred to as the active center. In the polymerization scheme, there are three main steps: initiation, propagation, and termination. As will be expanded on later in the chapter, the initiation step is the activation of the active center when a free radical reacts with the carbon–carbon double bond. After initiation, the active center reacts with other double bonds, propagating the chain to form a polymer and transferring the free radical to a new active center. The termination step can then occur in several ways: (1) two active centers can react; (2) one active center and one free radical can react; (3) the active center transfers to another molecule; or (4) interaction with impurities or inhibitors. Chain transfer is another important mechanism that occurs in free-radical polymerization. This occurs when an active center collides with a molecule such as a solvent, initiator or monomer, transferring the free radical to the second species.
Free-radical polymerization can theoretically go to full conversion, but the interaction of free radicals with each other must be kept in mind. Eventually, free radicals will form covalent bonds with each other, stunting the chain propagation and preventing full conversion. As a general rule, the greater the free radical concentration, the shorter the chain length. The vis...