
- 259 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
Latex products that we use in everyday life have a great impact on health and lifestyle. This book gives a comprehensive overview of how raw materials are prepared for latex manufacture and how they are converted to products by modern latex dipping methods. Tools for how to solve production problems encountered, quality control and how to validate the processes used in the latex industry are thoroughly discussed and described.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Latex Dipping by David M. Hill in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Industrial & Technical Chemistry. We have over one million books available in our catalogue for you to explore.
Information
1 Raw materials
1.1 Polymer latex or polymer solution?
In general, polymer latices are the material of choice for dipping, rather than solutions, for the following reasons. Unless the polymer is of a low molecular weight (MW) or at a low concentration, polymer solutions are viscous, which will result in problems with flow and controlling pick-up, and are prone to air being entrained giving rise to bubbles and possibly holes in the finished product. If the solution is diluted to a lower viscosity, the finished rubber film will be thin and several dips will be required to give a useable product. Latices, on the other hand, can be of a high solids content – typically 45 to 60% – coupled with a much lower viscosity. Polymer solutions are usually based on an organic solvent, which can be flammable, toxic or both, whereas latices are water based. It is possible to obtain aqueous solutions of some polymers, but the constraints of solids content and viscosity still apply. Some products are dipped from polymer solutions – for example, polyurethane (PU) condoms and some electricians’ gloves – but for the reasons outlined above, the overwhelming majority of dipped elastomeric products are formed by dipping latex. The nomenclature used here follows that described by Blackley [1]. Natural latices are produced via metabolic processes in trees and other plants, synthetic latices are produced by emulsion polymerisation of the relevant monomers and artificial latices are produced by dispersing the polymer (however, it may have been produced) in water.
Not all latices, however, are suitable for dipping. The polymer has to be film-forming at the temperatures used in the process – that is, the individual polymer particles which comprise the latex must be able to lose their individual identity and fuse together into a coherent film. The subject of film-forming will be dealt with in more detail in a later chapter, but these requirements mean that there are relatively few latices that are used commercially in dipping. Those in significant use are:
- – Natural rubber (NR).
- – Acrylonitrile-butadiene rubber [‘nitrile’ rubber (NBR)] and carboxylated nitrile rubber (XNBR).
- – Polychloroprene (CR) or chloroprene rubber. ‘Neoprene’, the trade name of DuPont’s CR, is in general use for this material.
- – Styrene-butadiene rubber (SBR) latex, although widely used in applications such as carpet backing and foam rubber, it is rarely used in dipping applications, although it can be incorporated into other latex formulations as a reinforcing filler.
There are also some artificial latices which are suitable for dipping and include:
- – Synthetic cis-1,4-polyisoprene rubber (IR) – the synthetic version of NR latex.
- – PU rubber.
- – Isoprene-isobutylene rubber (IIR) (‘butyl’rubber).
- – Ethylene-propylene diene monomer rubber (EPDM).
- – Chlorosulfonated polyethylene rubber (CSR).
Of these artificial latices, only the IR latex is currently being used to a significant extent in the dipping industry.
There are many other types of latices but none is used commercially to produce dipped rubber articles, although as mentioned above, SBR latex can be used as a reinforcing filler, as can polymethyl methacrylate (PMMA) latex.
1.1.1 Natural rubber latex
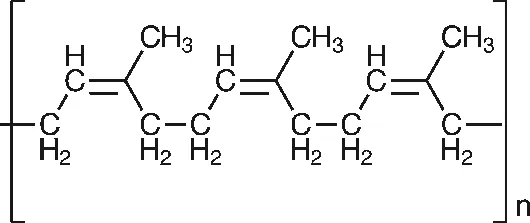
NR latex is a high molecular weight (up to 106 daltons) polymer of cis-1,4-polyisoprene, produced by the rubber tree Hevea Brasiliensis, which as the name implies, is a native of South America. to Kew Gardens in London towards the end of the 19th century. Seeds of the rubber tree were taken by the British explorer Sir Henry Wickham from Brazil Some of the seeds germinated, and seedlings were then transferred to Sri Lanka and Singapore, starting the cultivation of rubber in South- East Asia. Successful cultivation of the tree requires a tropical environment, with high temperatures and high annual rainfall. It is usually grown at lower levels and is rarely seen above 400 m. Intensive study over the years has resulted in the development of many different clones of the original strain, selected for high yield, rapid growth and resistance to disease. The structure of the NR molecule is shown in Figure 1.1.
Although NR is grown in many countries nowadays, at the time of writing the main production areas are in South East Asia, principally Thailand, Indonesia and Malaysia, Vietnam and India. There is also significant production in China, Sri Lanka, the Philippines and Cambodia, with smaller amounts in Africa (principally Liberia) and Central America. The production of NR latex in its original home in the Brazilian rainforests is still low, in part because of a fungal infection, South American leaf blight, to which the rubber tree is prone.
NR latex is present in the tree in specialised cells known as latex vessels, located just behind the outer bark of the tree. Synthesis of the polymer by the tree follows a complicated biological pathway and is not the result of isoprene monomer polymerisation. Instead, naturally occurring carbohydrates are converted via a complicated biological process into isopentenyl pyrophosphate, an important intermediary in many biosynthetic pathways. Isopentenyl pyrophosphate can, under certain conditions, form polyisoprenes via a condensation polymerisation. Detailed descriptions of the biosynthesis of NR in the rubber tree can be found in Natural Rubber Science and Technology by Roberts [2] and Polymer Latices Science and Technology by Blackley [3].
Latex is collected from the tree by the tapping process. A cut is made through the bark into the latex vessels, causing the latex to bleed out into a cup (Figure 1.2). It is important that the cut is not made too deep into the tree, as this would damage the tree and cause the bark to regrow irregularly, making subsequent tapping more difficult. Specialised knives are used to make the cut, and the operation requires a certain amount of skill to carry it out correctly.

As the latex vessels spiral clockwise up the tree, the cut will generally be made diagonally downwards, from high on the left-hand side to low on the right-hand side. This way, the maximum number of latex vessels are opened up. The latex flows out readily at first, then slows down and eventually dries up. Tapping is normally carried out early in the morning when the flow of latex is highest and temperatures (and hence the rate of drying) are lower. The yield from each tree depends on many factors, including the particular clone, the size of the tree and the age at which it was first tapped, amongst others. The trees will be tapped again, reopening the cut by shaving off as little of the bark as possible. Although some clones can be retapped daily, retapping generally takes place on alternate days, as the quality of the latex can be reduced by more frequent tapping. The latex will coagulate and dry if left, and is also prone to microbiological spoilage. For this reason, it is usual to put some ammonia into the collecting cup which prevents putrefaction and helps keep the latex liquid. The tapper will return to collect the latex from the collecting cup a few hours later and add it to the latex collected from the other trees. The bulked latex is transferred to the collecting station and from there onto the concentration factory. On arrival at the factory, the latex will be analysed to establish its quality, which will determine whether it is used to produce latex concentrate or dry rubber. Typically, this field latex will have a rubber content of around 30 to 35%. For subsequent use, the latex is concentrated to give a dry rubber content of about 60 to 65% – above this the viscosity rises rapidly and the latex becomes increasingly difficult to handle and process. The rubber particles have a density of around 0.92 kg/m3 and so are lighter than the aqueous phase in which they are dispersed. This density difference allows the latex to be concentrated by centrifuging. Latex can also be concentrated by creaming or evaporation, although most latex used for dipping is centrifuged. C...
Table of contents
- Cover
- Title Page
- Copyright
- Preface
- Contents
- 1 Raw materials
- 2 Latex compounding
- 3 Vulcanisation
- 4 Degradation of elastomers
- 5 Reinforcement of latex systems by the use of fillers
- 6 Latex stability and film-forming
- 7 Latex dipping
- 8 Wetting
- 9 Latex dipping tanks
- 10 Formers for latex dipping
- 11 Finishing operations for dipped latex products
- 12 Troubleshooting in latex dipping
- 13 Quality control in latex dipping
- 14 Process validation
- 15 Some analytical methods used in latex dipping operations
- 16 Allergies, proteins and N-nitrosamines
- 17 Potential future trends in the latex dipping industry
- Abbreviations
- Index