![]()
CHAPTER 1
Introduction
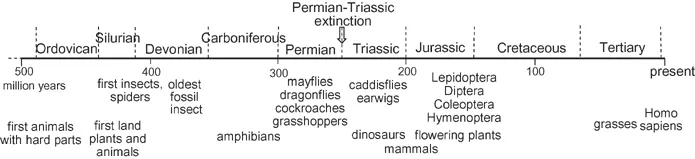
It is now over 420 million years since land plants first appeared on our Earth, at the end of the Silurian Period, and the first insects appeared about the same time. The oldest known fossil insect, Rhyniognathus hirsti, is between 396 and 407 million years old, from the beginning of the Devonian Period. There was a massive wipe out of insects in the Permian-Triassic extinction about 252 million years ago, but when flowering plants developed 200 million years ago, many Hymenoptera, Lepidoptera, Diptera and some Coleoptera evolved with them (Grimaldi and Engel, 2005). Insects have therefore branched off from the rest of the animals a long time ago, and have been evolving for much longer than mammals (Figure 1.1). Therefore a much greater diversity in genetic inheritance can be expected among insects than among mammals. Estimated numbers of insect species vary from one million to thirty million, making up 80% of all world species. New species are discovered at the rate of about 5000 per year. At any time, it is further estimated there must be some 1018 individual insects alive.
Figure 1.1 The antiquity of insects. The scale is in millions of years. Details vary considerably between authors. This figure is based on information in Grimaldi and Engel (2005).
Potentially, the subject of this book is gigantic but, at present, knowledge of insect biochemistry is but fragmentary.
All living organisms share much of primary or basic metabolism. The production of nucleic acids, amino acids, sugars, lipids and macromolecules made from these are processes common to all. Secondary metabolism is carried out by all organisms, but differs with different groups or species. Primary metabolism is sometimes defined as comprising all the reactions and compounds essential to the life of the cell, while secondary metabolism is the system of processes and compounds necessary to maintain the life of the whole organism. Secondary metabolites may be structural material like bone, chitin or hair, antibacterial or antifungal protectants, they may give protection from predators, they may produce warning or camouflage pigments, they may be used in signalling, such as hormones or pheromones, or they may have some function not yet identified. The division into primary and secondary metabolites is rather arbitrary and is rejected by some modern authors. It stresses the old idea of one enzyme-one metabolite, whereas one enzyme may catalyse changes in several or even many compounds (Firn and Jones, 2009).
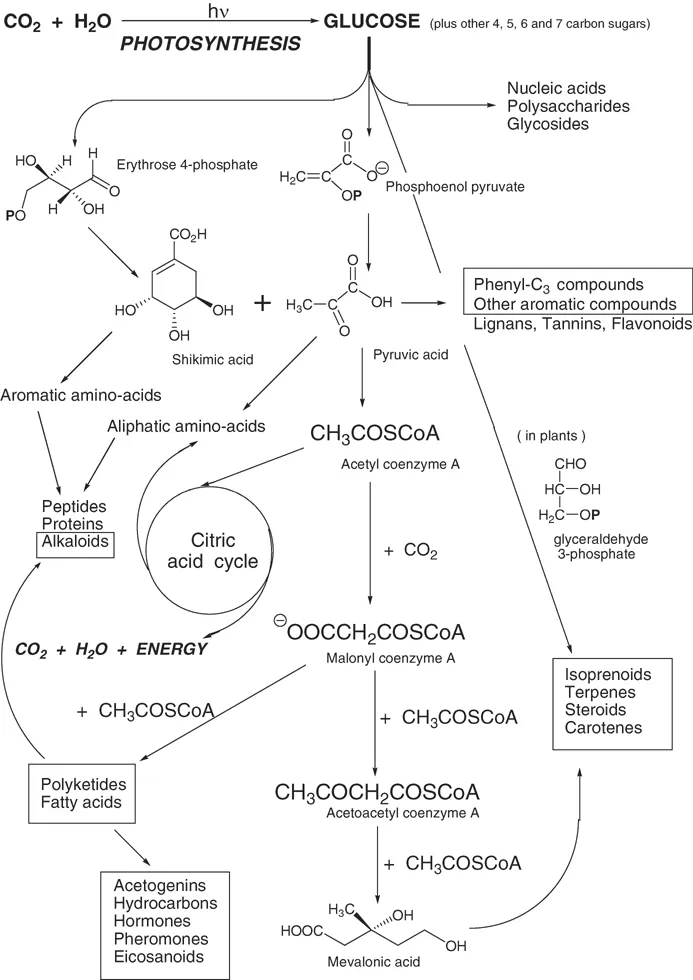
The range of secondary metabolites is enormous and presents a never-ending source of curiosity and challenge. Yet this great array of substances is produced from relatively few basic building blocks that are part of primary metabolism. Figure 1.2 attempts to summarize, very briefly, the basic groups of compounds from which secondary metabolites are made. The carbon atoms of all substances, whether from plant or animal, are ultimately derived from carbon dioxide via photosynthesis. It is remarkable that most compounds we consider here come through a very limited number of precursors. Figure 1.2 shows that many groups of compounds are formed via relatively few biosynthetic paths. Biosynthesis is the building up of chemical compounds through the physiological processes that take place in living animals, plants and micro-organisms. Biosynthesis requires starting materials, energy and catalysts. The starting materials may be any compounds from primary or secondary metabolism. All the compounds down the centre of Figure 1.2 are important starting materials for insect biosynthesis. The energy needed comes ultimately from sunlight, but is often immediately supplied through the breakdown of adenosine triphosphate, and the reactions are catalysed by enzymes.
Figure 1.2 A summary of the chief biosynthetic routes to primary and secondary metabolites. Those of particular interest here are enclosed in boxes. Notice that glucose, phosphoenol pyruvate and erythrose-4-phosphate are the key intermediates for all these classes of compounds. P indicates a phosphate ester. Adapted from a figure in J. Mann, Chemical Aspects of Biosynthesis, 1994, by permission of Oxford University Press.
Insects share some biochemical characteristics with all living organisms, others with all animals, but others are peculiar to insects alone, or to a few species or even a single caste of a single species (like males or queens of honeybees). In the words of Jerrold Meinwald and Thomas Eisner (1995), pioneers in insect chemical ecology, “The ability to synthesize or acquire an extremely diverse array of compounds for defence, offence and communication appears to have contributed significantly to the dominant position that insects and other arthropods have attained”. The compounds that insects produce are therefore a challenge to our ability to understand their structures and functions. The groups of compounds that are of special interest in the study of insects are enclosed in boxes in Figure 1.2.
The diverse secondary metabolites indicated in Figure 1.2 are called natural products by chemists. Though varied in their chemical structure, they are all made by one of these few biosynthetic pathways (in some cases, a combination of more than one of them)(Hanson, 2003). By understanding their biosynthetic origins one can make some sense of this multiplicity of natural products and group them according to their origin. Moreover, through understanding the mechanism of these biosynthetic reactions, and identifying the enzymes that catalyse them, and the genes that code for them, greater insight is gained into how these reactions and compounds might be manipulated in beneficial or pest species, to our advantage and to control or preserve the environment. In the past decade the information gained through the study of genes has leapt ahead, and we can expect genetic studies to transform this science in the years to come.
1.1 STUDYING BIOSYNTHETIC PATHWAYS
When considering the formation of naturally occurring substances, whether by plants, animals or micro-organisms, it should be remembered that all the reactions involved follow the normal laws of chemistry. One of the fascinating areas of chemistry today is trying to understand how living cells carry out these reactions. How is it that reactions we find extremely difficult in the laboratory are accomplished efficiently and quickly at room temperature and near neutral pH inside cells? What kinds of organic chemical reactions are used in living cells?
The immediate answer to the first question is that nature has evolved enzymes, efficient catalysts that lower the energy of activation for these reactions, to make them proceed much more quickly. Enzymes became active catalysts through repeated accidental, evolutionary changes over time, so that now almost every step in biosynthesis and metabolism is catalysed by an enzyme. Whatever the apparent “magic” effect of enzymes, the reactions must still obey the laws of thermodynamics; the reaction will still be explicable in terms of electron push and pull, bond and charge movements, proximity effects, pH, concentration of reactants and catalysts, and will follow the rules of organic chemistry. No biochemical reaction can go forward if the overall energetics are unfavourable to formation of the product. To give a fuller explanation, it is helpful to consider the nature and function of enzymes and some of the co-enzymes that often function with them (Chapter 2).
Emil Fischer, at the beginning of the twentieth century, had isolated two enzymes called invertin and emulsin. Invertin hydrolysed only α-D-glucosides (sucrose is an example) while emulsin hydrolysed β-D-glucosides. From this and his knowledge of sugars he correctly deduced that these enzymes were asymmetrically constructed molecules; in modern terms, they are chiral. Biochemical reactions take place on the chiral surface of an enzyme, which makes an important distinction from solution chemistry. The first enzyme obtained in a pure, crystalline state was urease, in 1926. It soon became clear that it and other enzymes were proteins.
The energetics and kinetics of enzymic reactions are important to the biochemist, but are not essential to our understanding of what kinds of compounds are produced by insects. Nevertheless, living systems are not static. Schoenheimer and Rittenberg (1936) showed that when an animal was allowed to drink heavy water (D2O or 2H2O) for a few days, the fatty acids of that animal became labelled with deuterium. When normal water then replaced heavy water, the deuterium again disappeared from the fatty acids, showing that cells, and whole animals, are in a state of dynamic equilibrium.
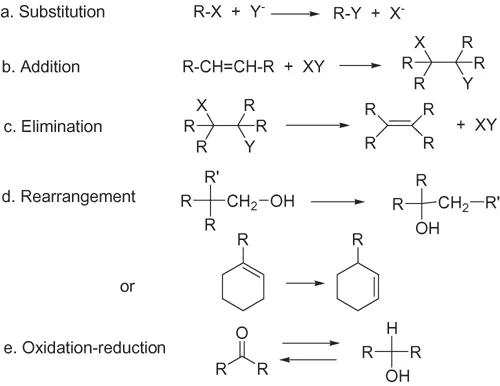
Organic chemical reactions that take place in living systems can be divided into five simple types, which are illustrated in Figure 1.3. Enzymes are known that catalyse all these types of reactions, but that simplicity is hidden in the great variety of enzymes and the individual reactions they catalyse.
Figure 1.3 The essential types of organic biochemical reactions.
1.2 PLANT VERSUS INSECT BIOSYNTHESIS
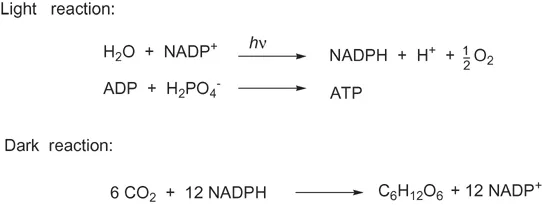
Plants have the ability to make a much greater diversity of compounds than animals. Above all, plants can use photosynthesis, splitting water in the light reaction, and in the dark reaction creating carbohydrates from carbon dioxide and hydrogen (Figure 1.4). Plants (and micro-organisms) have exclusive access to the shikimic acid pathway (Chapter 9), the aromatic amino acids and the methylerythritol pathway to terpenes. The case of the polyunsaturated acids (Section 4.1.2) may be unclear, since at least 12 insects have been shown to be able to make linoleic acid, but linolenic acid remains a substance made only by plants. The sterols (Chapter 8) can be made by plants and higher animals, but not by insects. The formation of carotenes (Chapter 8) by insects is doubtful. Compounds such as chlorophyll, starch, cellulose, lignin, tannins, anthocyanins, flavones and triterpenes can be made only by plants.
Figure 1.4 An outline of the reactions of photosynthesis in green plants. For NAD+, NADH, ADP and ATP see Chapter 2.
On the other hand, all the general biosynthetic methods used by insects, discussed in this book, are available to plants. The fatty acids and their derivatives, such as hydrocarbons (Chapter 4), the acetogenins (Chapter 6) and especially the terpenes (Chapters 7 and 8) and aromatic compounds (Chapter 9), can all be made by plants. Acetogenins are not as prominent among plant products as the others, except in the special case of formation of anthocyanins and flavones. Only special areas are left to insects alone. It is surprising to find, when compounds can be made by both plants and insects, that often they have found similar or the same way to biosynthesize them. This is sometimes attributed to parallel evolution, but the future may reveal other ways in which they share processes.
Plants, in their constant struggle with insects, have produced both physical defences (hairs, spines and thick waxy surfaces) and chemical defences (stinging trichomes, alkaloids, toxins and feeding deterrents) against insects, while insects are constantly evolving ways to overcome them. An interesting example of plant counter-attack are the phytoecdysteroids, made by plants, which mimic the natural moulting hormone of insects and may disrupt normal development of the insect feeding on them (Chapter 7). There are plant anti-juvenile hormone compounds too. Nevertheless, there is probably not a single plant species without at least one insect that has found a way to overcome its defences. A subject of growing interest is the production of volatile compounds by plants that have been attacked by insects that signal this to other plants and to parasitoids of the attacking insects (Section 5.9).
1.3 ARTHROPODS AND INSECTS
The arthropods were probably the first organisms to emerge from the sea, and insects were the first invertebrates to fly. Arthropods, nematodes and some smaller, less familiar groups are sometimes known as the Ecdysozoa, or moulting animals. The arthropods can be sub-divided into Crustacea (crabs, lobsters, shrimp, barnacles and woodlice), Chelicerata (spiders, ticks, mites, scorpions and others), Hexapoda or Insecta, and Myriapoda (millipedes, centipedes and other minor groups), although different authors use different classificat...