
- 131 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
The author illustrates why the rather weak hydrogen bond is so essential for our everyday life in a lively and entertaining way. The chemical and physical fundamentals are explained with examples ranging from the nature of water over the secret of DNA to adhesives and modern detergents. The interdisciplinary science is easy to understand and hence a great introduction for chemists, biologists and physicists.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access The Hydrogen Bond by Aloys Hüttermann in PDF and/or ePUB format, as well as other popular books in Scienze fisiche & Biofisica. We have over one million books available in our catalogue for you to explore.
Information
1 Before getting started
This book deals with what is called hydrogen bonding.
The word “bond” implies or means that something “comes together”. In a broader sense, chemistry is basically all about bonding. A hydrogen bond is a bond between molecules. To understand this in greater detail, we first need to take a look at how bonds work to begin with.
I am going to start with the very basics – but don’t worry, it’s not that hard to understand! So, if what I am about to tell you is something you already learnt in school, feel free to go straight to the next chapter.
Before I get into bonds, I would like to talk about the “building blocks” that are capable of forming bonds in the first place. The essential building blocks in chemistry are elements. You have probably heard of that. These elements basically represent letters of a chemical alphabet, meaning all existing compounds are made up exclusively of these elements, just like all words of our (written) language are made up of letters of the alphabet.
Just like all letters have a name – namely “A”, “B”, “C” and so on – every element has a name and a symbol that are used worldwide. Most of them are derived from Latin or Greek. For example, silver is called “Ag” (short for “argentum” = Latin for “silver”) and hydrogen “H” (short for “hydrogen” = Greek for “water-former” – which is a good name, because water actually contains hydrogen). While many metals have been known and named since ancient times – mostly metals such as iron, silver and gold – most of the other elements were named by those who discovered them. For a long time, names were chosen based on a property of the element, such as in the case of hydrogen. Oxygen, for example, has the symbol “O” for “oxygenium” = “acid-former”, because it is found in many acids that were known in those days. It was thought that the fact that acids are acidic was due to the presence of oxygen. Although this turned out to be incorrect, the name was left unchanged.
Naming conventions changed slightly over the years and, mainly in the nineteenth century, many elements were named after cities or countries, usually the country of the person who discovered the element. This includes “Ga” (short for “gallium” = France) – right next to “Ge” (short for “germanium” = Germany). France is represented twice, by the way, because there is also “Fr” = francium. The element “Po” = polonium gained unfortunate fame due to the poisoning of Russian Putin critic Alexander Litvinenko in 2006. By contrast, no elements were named after Great Britain and the United States during that period (which I find surprising), while the small Swedish village of Ytterby inspired the names of as many as four elements because of the minerals found in the nearby Ytterby mine: yttrium “Y”, ytterbium “Yb”, terbium “Tb” and erbium “Er”.
Today, more than 100 chemical elements are known, but only about 90 of them are stable enough to be used in chemistry on a larger scale. The other elements are radioactive and decay within a short period of time. But, of course, they are still given a name and a symbol! Apart from the tradition of naming elements after cities or countries, the names of famous scientists have established themselves as a particularly popular choice. That is why, alongside elements such as americium “Am” (America did get the honour, after all), californium “Cf”, berkelium “Bk” (named after the Californian university town of Berkeley) and its German counterparts darmstadtium “Ds” as well as hassium “Hs” (for the German state of Hesse), there is also einsteinium “Es” (after Albert Einstein), curium “Cm” (after Marie Curie) and roentgenium “Rg” (after Wilhelm Röntgen), among many others. The most recently named element is oganesson “Og” (after Juri Ogenasjan, one of the co-discoverers), discovered at the Joint Institute for Nuclear Research in Dubna, Russia.
All these elements are listed in what is called the “periodic table of elements”, because many properties of the elements repeat periodically.
More than 90 elements used in chemistry are metals, such as iron or copper. The chemistry of these elements is (usually) called “inorganic chemistry”.
Only a small number of elements, about ten, are of particular interest for this book, with the most important ones being carbon, hydrogen, oxygen and nitrogen. They have the symbols C (for carbon), H (for hydrogen), O (for oxygen) and N (for nitrogen). The chemistry of these elements, specifically that of carbon, is (usually) called “organic chemistry”.
Dating back to a time when chemistry was still relatively young and many things were not yet fully understood, the terms “inorganic” and “organic” are actually outdated. But since they turned out to be rather practical, they continue to be used. It was long believed that “organic” material, namely, molecules that can be obtained from living organisms such as animals or plants could not be synthesised from “dead” or “inorganic” materials such as minerals. The formation of organic materials was thought to always require a kind of vital force called “vis vitalis”, allowing organic materials to form only from other organic materials. However, in 1828, German chemist Friedrich Wöhler was able to demonstrate that this was not the case by producing an organic material, urea, from inorganic starting materials. There is no essential difference between organic and inorganic materials; a “vis vitalis” is not required. But since minerals usually do have a different composition than compounds that are found in animals or plants, these terms continue to be used for classification.
But there is no need to go into this any further. Instead, let us start with this essential question: Why do elements form bonds, anyway?
That is a good question, because actually there are elements that hardly form any bonds and are “happy by themselves”. These elements are noble gases and are called helium, neon, argon, krypton and xenon. These noble gases exist in the form of atoms, which means “alone”. They hardly form any bonds at all, not even with themselves (hence the name “noble”). Instead, the individual atoms in every noble gas occur in the form of balls that are floating around at different speeds depending on the temperature. But every atom is completely independent and has nothing to do with the others. Only when noble gases are cooled to a very low temperature do they turn liquid due to the slower movement and several other effects, which we need not discuss any further. But a noble gas has to get very cold for that to happen. Helium, for example, only turns liquid at –268 °C (−450 °F).1 That is only 5 degrees above absolute zero! The melting point of neon, the next larger noble gas, is at a “milder” −246 °C (−410 °F) and that of argon is at –186 °C (−303 °F). Due to these properties, it took a long time for noble gases to be discovered, despite the fact that argon makes up almost 1% of the atmosphere, making it more than a thousand times more common on the Earth than silver or gold! The discoverers of argon thought it was a bit “inert” because it does not react (Greek: argos). The other Greek-derived names of the noble gases also attest to the fact that they stand apart from the other elements: krypton is derived from kryptos = hidden, neon from neos = new, xenon from xenos = foreign. Helium is derived from helios = sun, because helium had already been confirmed to exist on the sun by 1868 and would not be discovered on the Earth until much later, in 1895.
So, how come noble gases hardly ever form bonds?2 It is because of their internal structure.
An element can be distinguished from another by the number of negatively charged electrons and positively charged protons. If nothing else has happened to the element, the number of electrons and protons is identical. The simplest element is hydrogen with one electron and one proton, followed by helium with two electrons and two protons, and so on. The protons are located in the atomic nucleus along with the neutrons, which are not relevant in this case, while the electrons are in the shell that surrounds the atomic nucleus.
The elements are arranged in what is called the “periodic table of the elements” in order of increasing atomic number, comparable to the way letters are arranged in the alphabet.
It has been discovered that certain numbers of electrons are special, namely 2, 10, 18, 36 and 54. Elements with these numbers of electrons have special chemical stability. Why is that?
Using a model that is relatively simple but perfectly sufficient for this purpose, you can visualise how electrons are not simply present in the atomic shell, but distributed across several shells that resemble the layers of an onion. Whenever a shell is full, there is great stability.
The shells can hold different numbers of electrons. The first shell closest to the atomic nucleus can hold two electrons, the next two shells can hold eight electrons and the two after those 18, with the shells being filled from the inside out. Explaining why exactly it is that way would be going too far. All in all, we can basically visualise it as follows:
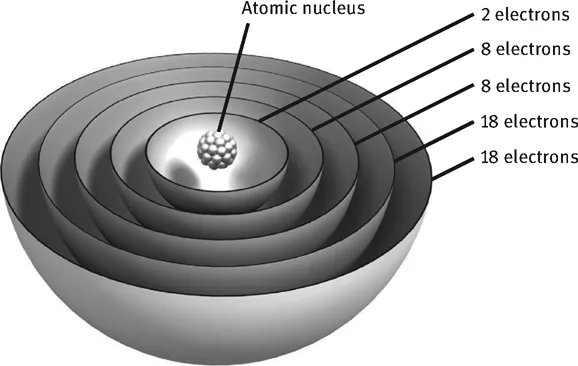
It is not by chance that noble gases have these “magic numbers” where the electrons exactly fill the shells! Helium has two electrons (i.e. the first shell is full), neon 10 (i.e. the first two shells are filled), argon 18 (the first three), krypton 36 (the first four) and xenon 54 (the first five).
The other elements that do not have these numbers of electrons are therefore often “tempted” to also reach a condition where one of these “magic numbers” is present – they form bonds.
How can this happen? There are basically three different possibilities:
1.1 An atom loses or gains electrons
This type of bond is found in sodium chloride, which is the salt you put in your food. Chemically, salt consists of two elements, namely sodium and chlorine.
As shown in the periodic table, sodium has 11 electrons, which is one more than neon. While sodium’s two innermost shells are completely filled, there is only one single electron in the third shell. But this does not mean that sodium is unstable. On the contrary, sodium is essentially a stable metal.
However, having ten electrons would make it even more stable, as we have seen earlier. That is why sodium “strives” to lose one electron to reach neon’s “magic number” of stability. Well, sodium does not strive, of course, because it has no will of its own....
Table of contents
- Cover
- Title Page
- Copyright
- Preface
- Contents
- 1 Before getting started
- 2 The “bond” in hydrogen bonding
- 3 The “water” in hydrogen bonding
- 4 The order in the ice
- 5 Dissolving like sugar in water
- 6 Soaps and cells
- 7 Recognise your vis-à-vis
- 8 Jerry Donohue and the DNA
- 9 How to make hydrogen bonds visible
- 10 “Now let’s step on the accelerator”
- 11 Substances that build themselves
- 12 Like Avalokiteśvara and Durga
- 13 Conclusion and acknowledgements
- Bibliography
- Index