![]()
Part I The Main Classes of Metal-based Anticancer Agents and their Modes of Action
![]()
CHAPTER 1
Enhancing the Therapeutic Potential of Platinum-based Anticancer Agents by Incorporating Clinically Approved Drugs as Ligands
Reece G. Kenny a and Celine J. Marmion*a
a Centre for Synthesis and Chemical Biology, Department of Chemistry, Royal College of Surgeons in Ireland, 123 St. Stephen's Green, Dublin 2, Ireland
*E-mail:
[email protected] In this chapter, we have endeavoured to showcase how some clinically approved drugs may be exploited as potential ligands when designing new metallodrugs to treat cancer. Interestingly, while there is a sound rationale behind repurposing existing drugs, those to date that have been tethered to platinum(ii) and platinum(iv) centres have not been chosen for this purpose. Rather, they have been selected because these drugs, in their own right, have exhibited potent anticancer activities albeit some are in clinical use for other indications. This chapter will provide an overview of some interesting platinum(ii) and platinum(iv) complexes incorporating a selection of clinically approved drugs or derivatives thereof as ligands. These complexes may form the basis of a new drug class which may offer advantages over existing therapeutic regimens.
1.1 Introduction
Cancer is a multi-factorial disease which results from a myriad of genetic and environmental factors. It continues to represent a global health challenge not least because the global population is growing and ageing but also because access to information related to its prevention, early detection and treatment options, particularly in developing countries, is limited. The lack of provision of adequate medical and public health infrastructure are also contributory factors. 1 While there are different options to treat cancer, they typically include a combination of surgery, chemotherapy and/or radiotherapy. Immunotherapy, a form of treatment which stimulates our immune system to kill cancer cells, 2 and oncolytic virotherapies, 3 are also emerging as promising options to complement existing therapeutic regimens.
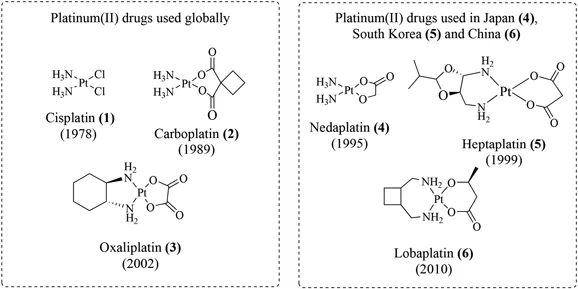
Chemotherapeutic drugs operate either alone or in combination regimens by blocking the unwanted proliferation of cancer cells. Platinum drugs constitute a major chemotherapeutic drug class for the treatment of cancer. In fact, it has been stated that nearly 50% of all cancer treatments involve platinum drugs. 4 Despite their clinical success, there are currently only three platinum drugs in worldwide clinical use, namely cisplatin (1), carboplatin (2) and oxaliplatin (3) and three others, nedaplatin (4), heptaplatin (5) and lobaplatin (6) in use in Japan, South Korea and China respectively (see Figure 1.1). 5 These square planar platinum(ii) drugs contain ‘non-leaving’ nitrogen donor ligands and labile chlorido or dicarboxylato ligands. Cisplatin, as a representative example, elicits its anticancer effect by first accumulating in tumour cells, whereupon the labile ligands are displaced by water ligands. It is these resulting aquated platinum(ii) species that can then irreversibly bind DNA (typically to the N7 of guanine nucleobases) leading to the formation of DNA lesions and ultimately triggering apoptosis or programmed tumour cell death. The reader is directed to a recent review which provides a comprehensive account into the development, mechanism of action and clinical utility of these ‘classical’ platinum(ii) drugs. 5
Figure 1.1 Platinum drugs in clinical use (1–6) with the years in which they received regulatory approval indicated in parentheses.
It is broadly acknowledged that these drugs are enormously successful against a wide range of cancers. They do however have significant limitations. While the lability of the anionic ‘leaving’ ligands is important for anticancer activity as mentioned earlier, it also means that the complexes are more susceptible to ligand substitution. Cisplatin, for example, can readily react with thiol-containing biomolecules such as glutathione and metallothioneins. These side reactions not only account for the toxicity profile associated with cisplatin and related complexes, from minor to dose-limiting, but also play a role in the emergence of resistance against platinum drugs. 6 For example, some cancer cells are known to overexpress thiol-containing biomolecules so even if the platinum drug accumulates successfully in tumour cells, those that contain high concentrations of these thiol-containing biomolecules compete with DNA for platinum drug binding. This leads to a reduction or elimination in the efficacy of the drug towards those cells. Of the platinum drugs in clinical use, all but cisplatin contain bidentate dicarboxylato ligands. These chelating bidentate ‘leaving’ ligands were chosen on the basis that they would confer greater stability on the complexes (chelate effect), enhancing their half-lives and thus reducing their susceptibility to these unwanted side reactions. The more stable carboplatin, for example, is significantly less toxic on the body than cisplatin. 5 In addition to limiting the formation of platinum-DNA adducts, cells have also acquired other modes in which to build resistance and reduce drug sensitivity to cancer cells. Such resistance can arise from multiple factors including genetic and epigenetic changes as outlined in a comprehensive review by Gottesman et al. 7 The reader is also referred to another relevant review on molecular mechanisms underpinning platinum drug resistance in ovarian cancer by Tapia et al. 6
In order to overcome toxicity and resistance issues associated with these ‘classical’ platinum drugs, a number of research approaches have been and continue to be actively explored. Early research focused on developing analogues of cisplatin, i.e. complexes in the cis configuration containing a central platinum(ii) ion, with ammine or substituted amine non-leaving group(s) and anionic labile ligands but none were found to offer any significant advantage over cisplatin. 8 Trans analogues 9,10 and polynuclear platinum(ii) drugs 11,12 have also been investigated with one trinuclear complex, BBR3464, 13 advancing to phase II clinical trials. This trinuclear complex represented the first example of a ‘non-classical’ platinum drug to advance to the clinical setting. This breakthrough undoubtedly prompted scientists working in this field to explore more ‘non-traditional’ approaches in their drug design strategies. There now appears to be a tangible shift in research focus which is resulting in a more ‘targeted’ approach in the quest to bring forward an alternative ‘classical’ or ‘non-classical’ drug class. The exploitation of nanotechnologies to selectively deliver platinum drugs to tumour cells is one such approach, which has received considerable attention of late. 5,14,15 An alternative approach is to tether targeting moieties on the platinum(ii) scaffold to generate complexes with greater selectivity for tumour cells and this may reduce unwanted side reactions and thus lower toxic side effects. 5 Developing complexes with a mechanism of action different to classical platinum(ii) drugs is another area being actively explored. These complexes may be able to overcome drug resistance issues that have been plaguing many therapeutic regimens including those related to platinum drugs. Alternatively, targeting drug transport mechanisms may lead to new complexes with an improved chemotherapeutic profile. 16 Another design strategy is to move from square planar platinum(ii) to the more kinetically inert, octahedral platinum(iv) complexes. Platinum(iv) complexes may offer advantages over platinum(ii) drugs in that the coordinatively saturated platinum(iv) centre is more resistant to ligand substitution. This may prevent the aforementioned unwanted side reactions with biomolecules and may thus increase the safety profile of these drugs. Furthermore, the presence of six coordination sites in octahedral platinum(iv) complexes, as opposed to four in square planar platinum(ii) complexes, allows for greater latitude in terms of adding extra functionality. For example, it is possible to incorporate bioactive ligands or targeting vectors in these axial positions. Upon uptake into the more reducing environment found in tumour cells, the bioactive ligands may be released following reduction of the platinum(iv) complex to its platinum(ii) analogue, a mechanism referred to as ‘activation by reduction’, and these ligands may then work synergistically with the resulting platinum(ii) agent in killing cancer cells. 5,17,18
Furthermore, given the risi...