![]()
CHAPTER 1
Introduction
1.1 AIMS
The aims of this chapter are to introduce the structure of medicinal chemistry and to show how the subject has developed. By the end of this chapter you should know the salient points concerning:
- the basis for the classification of drugs;
- the targets for the medicinal chemist;
- the stages in the development of a drug; and
- the history of medicinal chemistry.
Medicinal chemistry is concerned with the chemistry of compounds that have a beneficial effect on a disease. Its objective is to enhance this beneficial therapeutic effect of a compound by modifying its structure and to remove unwanted side effects through an understanding of the chemistry by which the compound exerts its biological effects. Once a lead substance with a useful biological effect has been discovered, the medicinal chemist must undertake a series of structural variations in order to establish a pattern of structure: activity relationships leading to an enhancement of the useful biological effect. While the medicinal chemist is primarily concerned with the synthesis of new therapeutic agents, there must be a considerable interaction with other disciplines; medicine and biology for the description of the disease state and the development of the bio-assay, pharmacology for the definition of the site of action and pharmacy for the delivery of the compound to the living system.
1.2 THE CLASSIFICATION OF DRUGS
Drugs may be classified in a number of ways. One way is in terms of the nature of the disease that they are being used to treat. Thus there are compounds that are used to treat infectious diseases. Second, there are compounds that are used to treat cancers and third, non-infectious diseases. The chemistry of infectious diseases is concerned with the development of drugs to injure an invading organism with minimal injury to the host. The targets are often the differences between the viral, bacterial, fungal or parasitic cells and those of man. This is the area of the antibiotics. The medicinal chemistry that is used in the treatment of cancers involves the use of drugs to destroy an aberrant cell within the host. The targets of cancer chemotherapy are the differences between the rapidly proliferating cancer cells and normal cells. Cancer chemotherapy is often used in conjunction with other forms of treatment such as radiotherapy. The chemistry of non-infectious diseases involves a study of the selective action of a drug on one cell or receptor in the host. In some cases the drugs are developed as mimics of natural hormones.
The drugs that are used in the treatment of non-infectious diseases can be further sub-divided in terms of their targets. There are a group of hormones known as neurotransmitters that are formed at nerve endings and convey the consequences of a nerve impulse to a receptor or an effector cell. There are drugs, which affect primarily the neurotransmitters in the central nervous system including the brain and the spinal cord. These include the psychotropic agents, the anti-depressants, hypnotics and analgesics. There are those agents, which affect the peripheral nervous system including the local anaesthetics. Another group of substances are those, which affect the circulatory system acting as anti-hypertensive and anti-thrombotic agents. These may interact with local hormones. These are a family of compounds that have a metabolic or endocrine target. These are compounds that are modifications of circulatory hormones that may be used as oral contraceptives or to correct a hormone deficiency. Finally there are those compounds, which target the immune system such as the immunosuppresive agents. However there is an overlap between these classes and a compound may show several types of biological activity.
The pattern of usage and the length of time over which a drug may be administered varies between the classes. Hence the extent to which side-effects can be tolerated varies quite widely. For example, an antibiotic may be given for a few days while a compound, which is given to correct a hormone deficiency or as an immunosuppresive agent may be administered for years.
1.3 TARGETS FOR THE MEDICINAL CHEMIST
1.3.1 Hormones as Targets
The body produces substances known as hormones, which regulate body functions. These can be circulatory hormones such as the steroid and peptide hormones. They are produced by one organ and are then transported to their target organ. Others, such as histamine are local hormones, which are produced by one cell and have their action on adjacent cells. These are sometimes known as autocoids. The third group are the neurotransmitters, which are formed and have their action at nerve endings. A fourth group are the ‘second messengers’. These are compounds that are formed within a cell often as a result of an external stimulus via a trans-membrane protein. They control the function of various enzyme systems within the cell.
Many hormones and neurotransmitters exhibit their cell-signalling biological activity by binding to a receptor on a cell surface. The receptor may be part of a trans-membrane protein, which crosses the cell wall. This binding to a trans-membrane protein then initiates a sequence of events within the cell. Other hormones have to cross the cell wall and exert their biological activity by binding to nuclear receptors within the cell. This activates the nucleic acids and initiates the DNA–RNA–protein sequence of events.
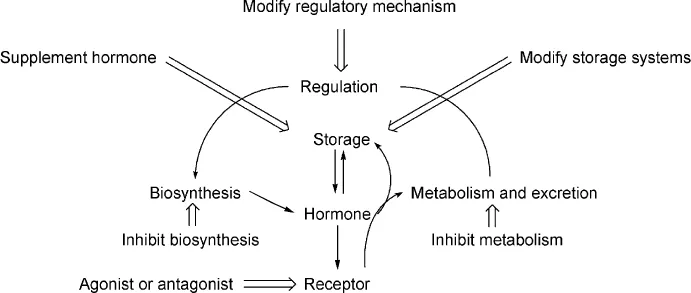
The hormones are biosynthesized by a series of steps and once they have produced their biological effects, they are metabolized and excreted. The medicinal chemist may interact with this sequence in a number of ways. The chemist may synthesize the biological compound itself and use it to correct a deficiency or an agonist may be prepared. An agonist is a relative of the naturally occurring substance that also binds to the receptor and elicits the same biological effect. A partial agonist is a compound, which binds but does not elicit the full response. In contrast to this an antagonist binds to the receptor site but does not produce the biological effect. It may block the effect of an agonist. Often agonists and antagonists have quite similar structures for both have to bind to the receptor.
The enzymes which mediate the biosynthesis of the naturally-occurring compound may be inhibited by a drug. Hence the hormone will not be formed and its biological effect will not be observed. Many enzymes are regulated by a metabolite from a later stage in the biosynthetic pathway. This type of feedback regulation may be used to moderate the amount of biosynthesis that occurs. The release of a compound from storage may also be a regulatory step. When enzyme systems are targets for drugs, the binding may be of a competitive and reversible nature or it may be irreversible. Sometimes the product of the reaction of the enzyme with an artificial substrate may then react with the enzyme itself preventing the enzyme from catalyzing further transformations. This type of inhibition is known as suicide inhibition.
Once a hormone or neurotransmitter has completed its biological function, it may be metabolized and excreted or it may participate in the feedback regulatory mechanism associated with its formation. If these later metabolic steps are inhibited, the action of the hormone may be prolonged. The re-uptake of a neurotransmitter may form part of the regulatory mechanism. Interferance with the re-uptake mechanism may also prolong the action of the neurotransmitter. These targets are summarized in Scheme 1.
Scheme 1 Hormonal targets for the medicinal chemist
1.3.2 Cellular Structures as Targets
Cellular structures and cellular constituents provide another series of targets for the medicinal chemist. The structure and formation of the viral, bacterial or fungal cell wall provide a series of targets. These cell walls have different structures to those of man allowing for selective action. If a cell wall cannot be formed correctly, it may rupture and the cellular constituents may leak out.
A cell wall has ion-channels through which ions can pass. The ion-channels, which allow sodium, potassium, calcium or chloride ions to enter a cell, form targets. The presence of these ions in the cell affects processes such as muscle contraction.
The interaction of a cell-signalling substance with a receptor can initiate a further series of enzyme-catalysed events within the cell. These enzymes may be the target for drugs. The nucleic acids form further targets particularly in cancer chemotherapy. Interferance with the biosynthesis of nucleic acids, their translation and replication, each form targets for drugs. Thus there are a plethora of potential targets for drug action. A crucial stage in the combat of a disease is the selection of an appropriate target. The selection of the target determines the bio-assay for a drug.
1.4 THE STAGES IN THE DEVELOPMENT OF A DRUG
The first stage in the development of a drug involves the establishment of a reliable bio-assay. This may be an antibiotic screen or a screen against a particular tumour cell line. It may involve enzyme or receptor assays. These days whole animal tests are rare in the primary screens although in previous years, they have played an important part. Nevertheless because of the complexity of biological systems, there are still situations in which whole animal tests have to be used to obtain information on a potential drug. Modern enzyme or receptor screens can be very rapid and have a high throughput allowing small amounts of many thousands of compounds to be screened within weeks. This in turn has led to new synthetic methodologies in organic chemistry such as combinatorial synthesis in order to generate a suitable range of compounds for testing.
There are a number of sources of lead compounds. These may be obtained by screening natural products particularly from plants that have been used in folk medicine. The lead compound may arise from random screening or from the clinical observation of a side effect of an existing drug. The rational design of a lead compound based on the structural modification of a hormone or an active site model, is an intellectually satisfying approach.
Once a lead compound has been identified, there is a progressive structural modification to enhance the activity and to identify the contribution of electronic and steric factors to the biological activity. This can be with the aid of computer based molecular-modelling techniques. The establishment of quantitative structure: activity relationships (QSAR) can lead to the identification of a part of the molecule that is responsible for the activity, the pharmacophore.
Drug metabolism studies and pharmacokinetic studies then follow. The identification of the metabolites of a drug can involve the medicinal chemist in the preparation of labelled material. Once a compound is under serious consideration as a drug candidate, animal toxicity studies are undertaken. On the chemical side the formulation and development of a manufacturing route and appropriate analytical methods have to be undertaken. Clinical studies then follow. The phase I trials involve healthy volunteers and aim to establish the acceptability of a compound in man and obtain some pharmacokinetic data, phase II trials are with a limited number of patients and aim to establish the efficacy of the drug. This includes proof of the principle underlying the activity. Finally phase III large-scale trials are used to establish the efficacy of a drug compared to its rivals. Evidence has to be obtained concerning the safety of the drug and any contra-indications for its use. During any one of these phases development may be stopped if toxicity is detected. The submissions to the drug safety and drug registration committees then follow such as the Medicines and Health Care Products Regulatory Authority and the U.S. Food and Drug Administration. Finally if a compound is to be prescribed in the National Health Service, its effectiveness has to be assessed and a recommendation made by the National Institute of Clinical Excellence (NICE). The current time scale between the initial programme and the release of a compound for use may be between 10 and 15 years and the cost may be of the order of £500 million pounds. The need for patent protection in these circumstances is obvious...