![]()
PART 1
Safety Studies: Human and Environmental Health
![]()
1
The State of the Science
BY STUART NEWMAN
Stuart Newman, PhD, is Professor of Cell Biology and Anatomy at New York Medical College and a founding member of the Council for Responsible Genetics. This article originally appeared in GeneWatch, volume 26, number 1, January–March 2013.
When scientists first learned in the late 1970s how to sequence DNA and transfer it from one kind of organism to another, improving foods and other crop plants by introducing foreign genes was among the first applications proposed. Given contemporaneous findings in molecular genetics, such as the recognition that a mutation in a single gene could promote a cell’s transformation to cancerous state,1 it was unsurprising that concerns were raised about the capability of the transgenic methods to dramatically change the biochemistry or ecological stability of plants. Some critics suggested that the quality and safety of fruits and vegetables could be impaired, making them allergenic or toxic to humans and nonhumans who consume them, or that “superweeds” might be created which could disrupt wild or farmed ecosystems.
By 2005, however, when more than 90 percent of the annual soybean crop and 50 percent of the corn crop in the United States had come to be genetically engineered—a transformation in agricultural production that took less than a decade2—efforts at testing and regulation of genetically modified (GM) foods were increasingly portrayed as irrational. A perusal of the summaries of recent policy articles on the PubMed database turns up dozens in which reservations about the massive introduction of GM food into the food chain are represented as scientifically ignorant, economically suicidal, and cruel to the world’s hungry. One abstract in the journal Nature reads: “Unjustified and impractical legal requirements are stopping genetically engineered crops from saving millions from starvation and malnutrition.”3
These papers—many by European commentators decrying the successful efforts to keep GM foods out of the markets there, and some by US commentators bemoaning the necessity to test these products at all—mainly support their cases by referencing short-term feeding studies of animals. But this type of study is not adequate to allay valid concerns. One group, reviewing the relevant areas, has written, “It appears that there are no adverse effects of GM crops on many species of animals in acute and short-term feeding studies, but serious debates of effects of long-term and multigenerational feeding studies remain.”4
According to another group that has looked into these issues:
Another team actually performed such long-term studies, with the findings that mice that were fed for five consecutive generations with transgenic grain resistant to a herbicide showed enlarged lymph nodes and increased white blood cells, a significant decrease in the percentage of T lymphocytes in the spleen and lymph nodes and of B lymphocytes in lymph nodes and blood in comparison to control fed for the same number of generations with conventional grain.6
A central issue for crop foods, of course, is their effects on humans. The most comprehensive review of this subject as of 2007 stated:
The same group revisited the literature four years later, reporting that whereas the number of citations found in databases had dramatically increased in the intervening period, new information on products such as potatoes, cucumber, peas or tomatoes, among others was not available. Regarding corn, rice, and soybeans, there was a balance in the number of studies suggesting that GM corn and soybeans are as safe and nutritious as the respective conventional non-GM plant, and those raising still serious concerns. They also note that “most of these studies have been conducted by biotechnology companies responsible [for] commercializing these GM plants.”8
Given the uncertainties of the long-term health impact of GM foods, it is significant that so far, virtually all genetic modification of food and fiber crops has focused on the economic aspects of production (i.e. making crops resistant to herbicides and insect damage, increasing transportability and shelf-life) rather than the more elusive goals of improving nutrition or flavor. Introducing biological qualities that enhance production, transportability and shelf life can compromise palatability, as seen with the Flavr Savr tomato, the first GM crop to be approved by the FDA for human consumption, two decades ago.9
To protect its investment against a skeptical public, the biotech food industry has depended on compliant regulators,10 on its proponents’ ridicule of biotech industry critics’ supposed scientific ignorance,11,12 and on expensive campaigns against labeling of prepared foods that would draw undue attention to the presence of GM components, which they claim to be natural and ordinary.13 (These are the same components that when presented to the Patent Office and potential investors are portrayed as novel and unique.) A food crop that actually benefited the people who eat it rather than only those who sell it would likely open the floodgates of greatly weakened regulation. Golden Rice, designed to provide Vitamin A to malnourished children, has failed to overcome the hurdles for approval for dietary use since it was first described in 2000. Though very limited in its ability to alleviate malnutrition, it has some merit in the prevention of blindness, and seems poised for approval in the next year or so.14 If so, it will almost certainly help agribusiness tighten its grip on the world food supply and increase its capacity to foist products that are much more questionable on their captive clientele—that is, everyone.
![]()
2
Antibiotics in Your Corn
BY SHELDON KRIMSKY AND TIMO ASSMUTH
Sheldon Krimsky, PhD, is the board chair of the Council for Responsible Genetics and professor of Urban and Environmental Policy and Planning at Tufts University. Dr. Krimsky served on the National Institutes of Health Recombinant DNA Advisory Committee from 1978–1981, chaired the Committee on Scientific Freedom and Responsibility of the American Association for the Advancement of Science, and has been a consultant to the US Congress Office of Technology Assessment. He is the author of numerous articles and books on regulation and the social and ethical aspects of science and technology. Timo Assmuth studied environmental sciences at the Universities of Turku and Helsinki, Finland, where he is affiliated as adjunct professor, and on a Fulbright grant at Tufts University Department of Urban and Environmental Policy and Planning in 2007. He has worked for twenty-five years in the Finnish Environment Institute, a government R&D organization, on wastes, soil protection, chemicals, GMOs, and environmental and health risks, with increasing interest in the human aspects of risk assessment and management. This article originally appeared in GeneWatch, volume 22, number 1, January–February 2009.
Biotechnology, including technologies based on genetic engineering or genetic modification, is becoming increasingly important in the global economy, ecology and politics. Agricultural biotechnology for food production has been the subject of much interest and debate in international politics, but in terms of market value, health care represents the largest sector of biotechnology, with pharmaceutical substances playing the major role. Most biotechnological pharmaceuticals are produced in microbes, but the use of genetically modified plants (often called “pharmacrops”) to this end has gained increasing attention.1 The first field trial permit for GM plants based on an application using the term “pharmaceutical” was issued in January 1991 in the US By the year 2006 there had been 237 applications for field trials in the US alone; however, no commercial products have resulted to date.2 Among the drugs being produced in plants are vaccines, antibodies, antigens, hormones, growth factors and structural proteins.
The possible advantages of plants over other systems in producing drugs include the production of larger volumes of drugs, more flexibility and cost-effectiveness in manufacture, better suitability of plant cells for production, and the potential of using plants and seeds for drug storage and delivery.3 Plants have also some safety advantages over other pharmaceutical production systems, such as safety from contamination with human pathogens, endotoxins and tumorigenic DNA sequences.4
On the other hand, pharmacrops present important new risks and safety issues. By definition, they are used to produce substances that have potent biological effects on humans and other higher animals. Pharmacrops contain higher concentrations of active substances than these animals are ordinarily exposed to in GM plants. Several genetic modifications are often carried out simultaneously, increasing risks.5 Risks arise not only from biological but also socio-economic factors.
Pharmacrops consequently introduce special challenges to regulation. This is inherent in their position between agricultural, medical and general industrial biotechnology, and the special ecological-physical and socio-technological characteristics of these technologies. Some of these regulatory challenges include:
• The extension of new-generation GM crops to novel processes wherein the plants are not intended to be utilized as food crops but rather as plant-based ‘drug factories.’
• Emergence of new forms of biopollution in possible gene transfers of GM pharmacrops to conventional crops.
• New methods required to evaluate drugs derived from plants that are grown in open fields.
• The need to align environmental, food and agricultural, as well as pharmaceutical and medicinal policies and regulatory procedures.
• The subsequent introduction of new actors, new interests and new contested issues regarding the development and application of the technology.
Risk issues are particularly urgent when pharmaceuticals are produced in plants that are potential food crops.6 The need to control these risks has been stressed, by both consumers and food processors.7 Currently pharmacrop risks are still addressed mainly within the conceptual frameworks of other GM food plants, and it is unclear how to accomplish the protection and management of food supplies as the distinction between food and pharmaceuticals becomes blurred.
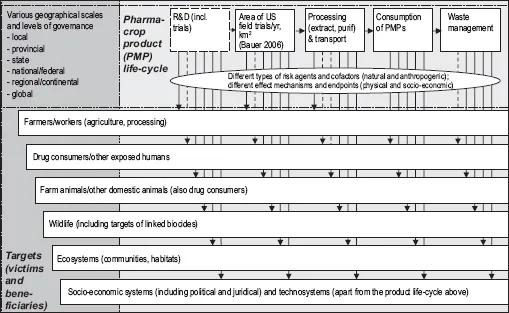
The main direct risks associated with pharmacrops can be categorized in terms of causative agents (for instance, the drug being produced); dispersal processes (especially gene flow) and environmental fate of the produce; exposed organisms or systems (such as animals which feed on pharmacrops in field trials); and biological (toxic, allergenic, ecological), agricultural and social effects. All of these need to be accounted for in the life-cycle of the technology (see Figure 1).
Figure 1. Risks along the cycle, to multiple targets. Many of these targets are routinely excluded from assessment.
Indirect risks arise in complex socio-ecological processes, also from attempts to control risks which inadvertently create new risks, for instance when using unproven ‘terminator’ technology to render GM plants sterile, combating vandalism by non-disclosure of information on field trial sites, or causing losses of relative benefits from pharmacrops as compared with conventional drug manufacture.8 Some risks may be irreversible, especially in regard to gene flow into the environment. Accidental outbreaks from field trials and associated food chain contamination scandals indicate that the transgenes cannot be totally contained.9 The crucial questions become how the different kinds of risks are judged and weighed against each other, what risks are deemed acceptable and on whose criteria, and what are feasible and justified risk abatement or prevention options.
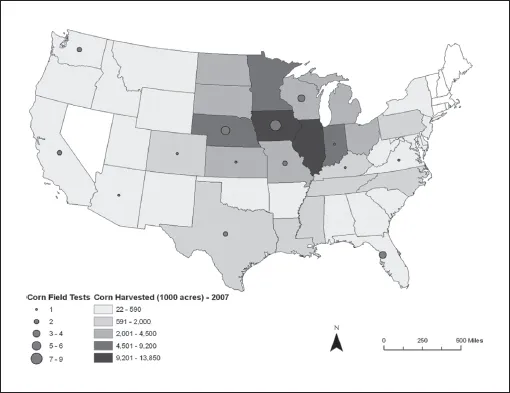
The risks of pharmacrops are unevenly distributed geographically. The map of field trials in the United States (Figure 2) shows that transgenic corn with pharmaceutical proteins has been tested mainly in the Corn Belt. Threats to food production systems, biodiversity, worker safety and rural development also vary according to location. If a pharmacrop is grown near fields of the same species, the risk of transferring the “drug gene” to a conventional crop is increased. The benefits are unevenly distributed as well; those who stand to directly benefit from a field test (such as pharmaceutical companies or landowners paid to allow test plots on their land) do not necessarily share the risks, and thus the presence of potential risks does not necessarily inform decisions such as location of field plots. For example, Iowa and Nebraska—two of the top corn producing states in the United States—have some of the highest numbers of corn pharmacrop test plots, despite the heightened risk of contamination or cross-pollination.
Figure 2. Pharmacrop corn field trials by state through November 2007 (APHIS data; map courtesy of Barbara Parmenter, Tufts University)
Castle (2008) singled out informed consent, risks to agricultural policy and intellectual property rights as the key global challenges for ethical production of vaccines in plants. The awareness, willingness (political) and capacity to respond to these issues varies between and within societies, and as a result there can be a mismatch between risks and responses. The geographical heterogeneity of risks and regulation increases from small nations to the US, the EU and the global systems.10
In the US, the policy toward pharmacrops has been relatively lax, but the regulatory procedures for assessing and managing their risks hav...