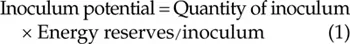![]()
1 Concepts of Epidemiology of Forest Diseases
Jonàs Oliva,* Johanna B. Boberg, Anna J.M. Hopkins and Jan Stenlid
Swedish University of Agricultural Sciences, Uppsala, Sweden
1.1 The Definition of Epidemiology
Epidemiology refers to the study of the occurrence and cause of infectious diseases, their origin, and their distribution in space and time within a population (Schumann, 1991). Its literary meaning refers to diseases among a human population, but the term is also widely used for the spread of diseases in plant populations. The term epidemic is often misused to describe only rapid and widespread diseases, but it should refer to the development of any disease, irrespective of its speed and extent (Tainter and Baker, 1996). Questions typically dealt with in epidemiological studies in an attempt to understand disease development are the following (Schumann, 1991):
• What kind of pathogen is causing the disease?
• Where did it come from and how was it dispersed?
• How will the disease spread, seen both from a short time (within a year) and a longer time (between years) perspective?
In forestry, infectious diseases are caused by a diverse range of organisms, including fungi, oomycetes, bacteria, phytoplasmas, parasitic higher plants, viruses and nematodes (Tainter and Baker, 1996). Among these, the fungi are the largest group causing disease in forest trees (Sinclair and Lyon, 2005). Different Phytophthora spp. belonging to the oomycetes group are also important as the causal agent of several devastating diseases on trees.
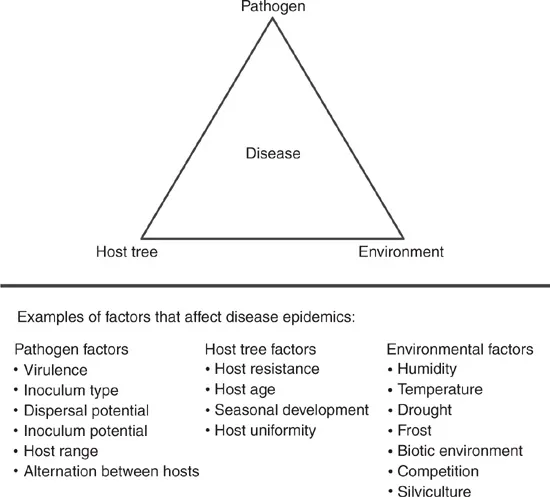
1.2 The Disease Triangle/Pyramid
Forest disease epidemics develop as the ultimate consequence of the interaction between a susceptible host plant, a virulent pathogen and a favourable climate. The interaction between these three elements of plant diseases, i.e. the host plant, the infectious agent and the environment, can be visualized and described by the disease triangle (Fig. 1.1) (Agrios, 2005). This conceptual model was first formalized by McNew (1960) and can be used to understand how epidemics develop and how they may be predicted and limited (Scholthof, 2007).
1.2.1 Pathogen factors
A pathogen has a basal set of pathogenicity factors that make it able to cause disease. It can have high or low aggressiveness depending on how efficiently it will cause disease. An aggressive pathogen can rapidly infect the host and produce large quantities of inoculum. Thus the aggressiveness of a pathogen is related to several factors; these include the quantity of inoculum produced, the mode of dispersal of the pathogen and the pathogen reproductive cycle. The form of the inoculum is also important as this can influence the speed with which the host is colonized.
Fig. 1.1. The disease triangle illustrates the interaction between pathogen, host and the environment as a prerequisite for disease to occur. The triangle may serve as a conceptual model describing the factors that influence the development of an epidemic. Also, included are examples of pathogen, host and environmental factors that may affect their interaction and favour disease and, thereby, the extent of disease epidemics.
The quantity of inoculum (bacteria, fungal spores, nematode eggs, etc.) is important for infection as high amounts increase the likelihood of a reproductive propagule successfully reaching and infecting a potential host. Knowledge about the initial amount of inoculum present (e.g. spores) is also important when describing disease spread using modelling or simulations. However, the potential for averting disease is not only dependent on the amount of spores. The concept of inoculum potential (Eqn 1), as defined by Garrett (1956a), acknowledges this and attempts to quantify the energy that is carried by the pathogen at the point of infection and is available to overcome host defences and initiate disease. Garrett stated that the inoculum potential may be increased either by increasing the quantity of inoculum (e.g. spores, individual mycelium) per unit area of host surface or by increasing the energy status of each unit. In his experiments using rhizomorph-producing Armillaria spp., Garrett (1956b) showed that the speed of infection by the rhizomorphs increased with the amount of inoculum and decreased with the distance between the inoculum source and the host. Subsequently, when the inoculum potential of the fungus was low, the infections were inhibited by host resistance.
Similarly, the closer the source of the inoculum is to suitable hosts, the higher the probability that the propagules will reach the host, which will also increase the chance of an epidemic. For example, spread from an infected tree to neighbouring trees in a forest is more likely than spread from the same tree to trees in neighbouring forests. The quantity of infective propagules that reach a specific host is known as the inoculum loading. When the inoculum loading, and thus also the inoculum potential, is very high hosts previously considered resistant to a pathogen may become susceptible. Foliar infections of Dothistroma spp. have been observed on the atypical host Norway spruce (Picea abies (L.) Karst.) for example, as a result of high inoculum loading from adjacent severely infected pines (Pinus spp.) (Lang, 1987).
In pathogenic fungi, several different types of inoculum are often produced. Spores, both asexual (conidia) and sexual, can be emitted in large amounts; they are often microscopic in size and are effectively spread in air, water or soil (Tainter and Baker, 1996), either directly or by vectors. Not only the nature of the propagule, but the way in which the reproductive propagules are produced, determines the speed and severity of epidemics. For example, spores produced on the outside of the host (such as on the bark of a tree or on its leaves) can be easily and effectively dispersed to new hosts. Other pathogens may use the host seed for reproduction and dispersal. Some pathogens produce propagules within the host plant material, so it is difficult for them to spread without the help of vectors. An example is Ophiostoma novo-ulmi Brasier, the causal agent of Dutch elm disease, which produces its spores within the wood of host elms (Ulmus spp.) and relies on bark beetles to collect and disperse the spores to new hosts (Brasier, 1991). Other pathogens produce inoculum and infective structures such as mycelia or rhizomorphs on infected plant parts within the soil, where they have a limited capacity of spread.
The mode of dispersal and the number of disease cycles per season are important factors in epidemics. Some pathogens have short reproductive cycles that allow several generations per year, while others reproduce less frequently. Propagules can be airborne, soil-borne or waterborne, or dispersed by host seeds or by vectors. Pathogens with airborne propagules are possibly the most important group as they can spread easily and cause sudden and widespread epidemics. The second most important group of pathogens have airborne vectors, e.g. O. novo-ulmi, and the pine wilt nematode Bursaphelenchus xylophilus Steiner & Buhrer, which are both spread by insect vectors. Pathogens spread by windblown rain can cause severe but localized epidemics, while when pathogens are seed dispersed, such as can occur for the pine pitch canker pathogen Gibberella circinata Nirenberg & O’Donnell ex Britz, T.A. Cout., M.J. Wingf. & Marasas (Storer et al., 1998), or spread in soil (like many Phytophthora spp.), they tend to cause localized, slow-spreading and severe diseases. In reality, many pathogens produce more than one type of propagule and so can use several different modes of dispersal, thereby increasing the possibility for epidemics. While spores are important for long distance dispersal of fungal pathogens, for example, other sources of inoculum, such as rhizomorphs and mycelia, may be very important locally. Spread via rhizomorphs and mycelia has a significant influence on the development of disease caused by decay fungi such as species of Armillaria and Heterobasidion (Woodward et al., 1998). Humans can also play a role in the dispersal of pathogens.
Following dispersal, there are a number of distinct steps that lead to the development and perpetuation of disease in a host. Together, these steps make up the disease cycle. The disease cycle can closely align with the life cycle of the pathogen, but it actually refers to the disease in the host as a function of the pathogen. The disease cycle can be broken up into four main phases: pre-entry, entry, colonization and dissemination. The pre-entry phase starts with inoculation: the first contact between propagules of the pathogen and the host. Following inoculation, the propagules start to grow on the surface of the new host substrate towards a penetration point. In the case of spores, the growth is divided into two stages: germination of the spores and the production of a germ tube. Favourable environmental conditions (such as suitable temperature and moisture) are required for these growth stages to occur. During the subsequent entry phase, the pathogen may enter the host by directly penetrating cells, by penetrating through natural openings (such as stomata) or by penetrating through wounds. The pathogen may form specialized structures such as appressoria or infection pegs to penetrate the host substrate.
During the colonization phase, the host is colonized by the pathogen, followed by the appearance of the first symptoms of disease, and then the production of spores and their dispersal. Colonization of the host can be intracellular or intercellular. Pathogens typically utilize one of two different strategies when colonizing their host: they act as either biotrophs or necrotrophs. Biotrophs obtain their nutrients from living cells, so keep their host’s cells viable during the colonization process. Typically, biotrophs produce infection structures and specialized organs for nutrient uptake and metabolite exchange, e.g. haustoria. Biotrophs often cause limited damage to the host, target fast-growing plants and have a limited host range. Rusts and powdery mildews are examples of biotrophs. In contrast, necrotrophs obtain their nutrients from dead cells. This means that they must kill their host cells before colonization. Unlike biotrophs, some necrotrophs have no infection structures and instead enter the host via wounds. They can cause severe symptoms and target weakened plants; they usually have a wide host range. The widespread forest pathogen Phytophthora cinnamomi Rands is an example of a necrotroph. It is also possible for pathogens to have a significant lag time between entry into the host and the development of disease symptoms, i.e. an incubation period. Frequently this occurs because the pathogen enters a dormant or latent phase; it then remains inside the host in a dormant stage, neither actively growing nor causing disease, until conditions within the host are suitable.
Disease cycles can be classified as either continuous (where the pathogen is found on one or one of several hosts throughout the entire disease cycle) or discontinuous (where the cycle is broken by a resting or non-pathogenic phase in the pathogen).
In continuous disease cycles, the unbroken disease cycle can take place on a single (primary) host, although sometimes part of the disease cycle can take place on an alternative or alternate host. An alternative host acts as an alternative if the primary host is not present. The alternative host is often closely related to the primary host and can show few or no disease symptoms. In contrast, disease cycles with an alternate host usually involve the pathogen completing part of its life cycle on that alternate host. This is usually an obligate part of the disease cycle or life cycle of the pathogen, and the alternate host is usually unrelated to the primary host. Many species of macrocyclic rusts, such as Cronartium ribicola J.C. Fisch., the causal agent of white pine blister rust, require the presence of the alternate host, in this case Ribes spp., to reinfect the primary host and cause widespread disease (Cobb et al., 2010). Some disease cycles include survival or overwintering of the pathogen when the primary host is not present or unsuitable for new inoculation and colonization. This is a critical step in the life cycle of the pathogen; therefore, if this phase is present in the disease cycle, it is often manipulated for control or management of the disease.
In discontinuous disease cycles, the pathogen can survive outside the primary host using one of a number of different mechanisms. One such mechanism is to form resting stages or survival structures. Many Phytophthora spp., for example, are able to form thick-walled chlamydospores that can survive for many years in the soil, waiting for suitable environmental conditions to germinate and infect a new host. Other examples of resting structures include oospores, teliospores, sclerotia and cleistothecia. Pathogens with a discontinuous disease cycle can also survive in a saprophytic phase in which the pathogen obtains nutrients from dead organic matter. Many leaf pathogens can survive as saprophytes on debris on the forest floor, and spores produced on the debris can then infect new hosts when environmental and host conditions are suitable. Finally, some pathogens survive outside their primary host by forming an epiphytic phase, i.e. they live on the surface of their host for some time without causing disease.
Most pathogens have a narrow host range, i.e. they are specialists that are specific to host species within the same genus, e.g. O. novo-ulmi, which causes disease only in elm trees (Brasier, 1991). Other pathogens are generalists and have a much broader host range; these can be exemplified by Phytophthora ramorum Werres, De Cock & Man in’t Veld and P. cinnamomi, which both infect plant and tree species belonging to a broad range of genera (Hansen et al., 2005).
1.2.2 Host factors
The host response to an invading pathogen contributes to determining the impact and severity of the resulting disease, and can range from extreme susceptibility to complete resistance. The susceptibility or resistance of the plant host can be age related (ontogenetic) (Develey-Rivière and Galiana, 2007) or organ specific (Blodgett et al., 2005). Resistance can be expressed constitutively or induced (Eyles et al., 2010) and can also be expressed systemically or locally. The resistance can be specific for a given pathogen (race-specific or vertical resistance) or general (basal or horizontal resistance) (Király et al., 2007).
Ontogenic resistance is related to the developmental stage of the host and is often observed as a decrease of susceptibility with host age. As an example, the susceptibility of Monterey pine (Pinus radiata D. Don) to needle blight caused by Dothistroma spp. in New Zealand is highest in young stands, up to 12 years old and then decreases (Watt et al., 2011b). The susceptibility of certain developmental stages to disease can also be related to environmental factors. Scots pine (Pinus s...