
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Advances in Alzheimer's Research: Volume 1
About this book
Alzheimer's disease (AD) is currently recognized as an untreatable, progressive, degenerative and terminal disease that is globally afflicting an estimated 36 million people and this number is growing in an unabated and frightening manner. Advances in Alzheimer Research, provides researchers with an integrated approach to AD academic literature ranging from basic to advanced clinical research. The series highlights the latest information in order to unravel the origin, pathogenesis and prevention of AD. The purpose of this book series is, therefore, to capture and discuss both, improvements towards the diagnosis and potential treatment of AD by established and novel strategies.
This first volume of the series provides an important mechanism to bring individuals having a variety of scientific interests and expertise under one roof to specifically focus on AD and related dementias. This volume presents articles on beta amyloid protein targets as well as research on secretase enzyme systems among other topics that deal with AD therapy.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
The What, Where, and How of γ-Secretase Complex Assembly
Daniel R. Dries*, †, Gang Yu*
Abstract
* Address correspondence to Daniel R. Dries and Gang Yu: Department of Neuroscience, The University of Texas Southwestern Medical Center, 6000 Harry Hines Blvd., NA04.508, Dallas, TX 75390-9111, USA; Tel: 214-648-5157; Fax: 214-648-1801; E-mail: [email protected] and [email protected]† Current Address: Juniata College, Chemistry Department, 1700 Moore Street Huntingdon, PA 16652, USA; Tel: 814-641-3557; E-mail: [email protected]
Introduction
Proteolytic Processing of the Amyloid Precursor Protein to AΒ
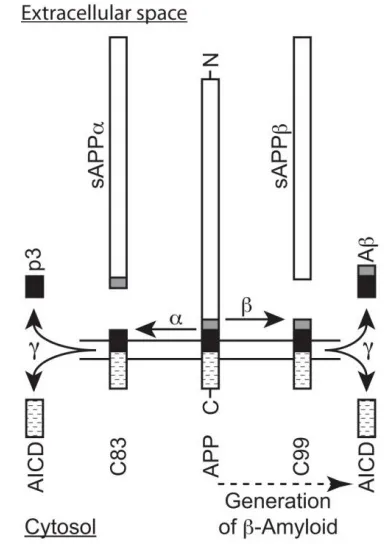
Table of contents
- Welcome
- Table of Contents
- Title
- BENTHAM SCIENCE PUBLISHERS LTD.
- PREFACE
- LIST OF CONTRIBUTORS
- How and When Environmental Agents and Dietary Factors Affect the Course of Alzheimer’s Disease: The “ LEARN ” Model (Latent Early-Life Associated Regulation) May Explain the Triggering of AD
- Therapeutics Targeting Intracellular Amyloid β-Protein in Alzheimer’s Disease: A Novel Effect of Apomorphine
- Protection Mechanisms Against Aβ42 Aggregation
- An Increase in Aβ42 in the Prefrontal Cortex is Associated with a Reversal Learning Impairment in Alzheimer’s Disease Model Tg2576 APPsw Mice
- The Regulation of βAPP and PrPc Processing by α-Secretase
- Regulation and Activation of Metalloproteinase-Mediated APP α-Secretase Cleavage
- Taking Down the Unindicted Co-Conspirators of Amyloid β−Peptide-Mediated Neuronal Death: Shared Gene Regulation of BACE1 and APP Genes Interacting with CREB, Fe65 and YY1 Transcription Factors
- The What, Where, and How of γ-Secretase Complex Assembly
- Pro-Inflammatory Cytokines and Anti-Inflammatory Drugs Modulate Glial Expression of Apolipoprotein E Protein
- Lipoprotein Receptors in Alzheimer´s Disease: Beyond Lipoprotein Transport
- Tau-Induced Neurodegeneration in Alzheimer Disease and Related Tauopathies
- Prevalence of Neuropsychiatric Symptoms in Alzheimer’s Disease and Vascular Dementia
- Fatty Aspirin: A New Perspective in the Prevention of Dementia of Alzheimer’s Type?