
Photoorganocatalysis in Organic Synthesis
- 600 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Photoorganocatalysis in Organic Synthesis
About this book
The use of organocatalysts able to photocatalyze an organic reaction is a rapidly growing field. These photocatalyzed transformations are more environmentally sustainable with respect to the use of expensive/toxic metal-based (photo)catalysts.
Based on the authors' extensive experience in photogenerated intermediates, this book presents an overview on photocatalyzed organic processes having a synthetic significance, where an organic molecule functions as the photocatalyst.
After a brief introduction defining the nature and the characteristics of a photoorganocatalyst (POC), the chapters are organized according to the class of POC used, as detailed below.
Each chapter begins with a summary of the photophysical characteristics of the POCs and is followed by selected examples of synthetic applications. The last two chapters are devoted to the adoption of photoorganocatalysis in polymerization and to flow photoorganocatalysis. These in-depth explanations and practical applications make this title an essential reading for any chemistry student interested in organic (sustainable) synthesis.
Contents:
- Ketones and Aldehydes (Shin Kamijo)
- Quinones (Akichika Itoh)
- Aromatics and Cyanoaromatics (Stefano Protti and Davide Ravelli)
- Sulfur Heterocycles (Katarzyna Goliszewska, Katarzyna Orłowska and Dorota Gryko)
- Oxygen Heterocycles: Pyrylium Salts (Sergio M Bonesi and Al Postigo)
- Oxygen Heterocycles: Eosin Derivatives (Wen-Jing Xiao, Xiao-Qiang Hu, and Jia-Rong Chen)
- Oxygen Heterocycles: Fluorescein, Rhodamine, Rose Bengal (Daniele Leonori and Fabio Juliá)
- Nitrogen Heterocycles: Porphyrins (Hermenegildo García and Sonia Remiro-Buenamañana)
- Nitrogen Heterocycles: Acridinium Salts (Peter Fodran, Leticia Monjas and Carl-Johan Wallentin)
- Other Nitrogen Heterocycles: Carbazoles, Imides and PDI, mpg-C₃N₄, Tetrazines, Riboflavin, and BODIPY (Pier Giorgio Cozzi, Paola Ceroni, Andrea Gualandi, and Marianna Marchini)
- Photoorganocatalysts for Polymerization (Didier Gigmes, Frédéric Dumur, Nicolas Zivic, Ségolène Villotte, Jason C Morris, Jacques Lalevée and Aude Héloïse Bonardi)
- Photoorganocatalysis in Flow (Manuel Anselmo, Andrea Basso and Lisa Moni)
Readership: Any chemistry student interested in organic (sustainable) synthesis.Organic Chemistry;Photochemistry;Photocatalysis;Organocatalysis;Reactive Intermediates;Radicals00
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Chapter 1
Ketones and Aldehydes
1.1Introduction
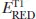
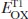
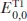
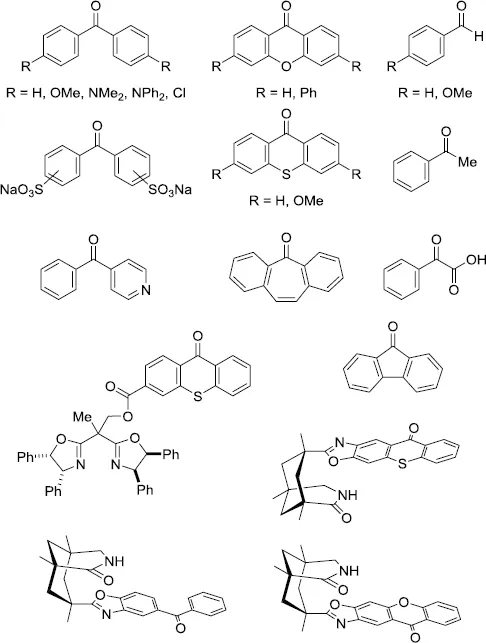
1.2Functionalizations of Unsaturated Bonds
1.2.1Alkylation of olefins
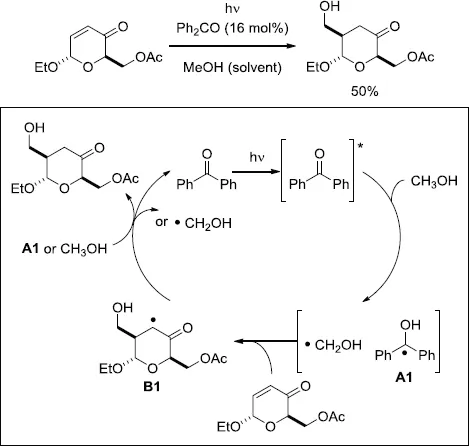
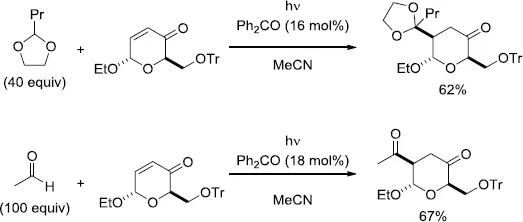
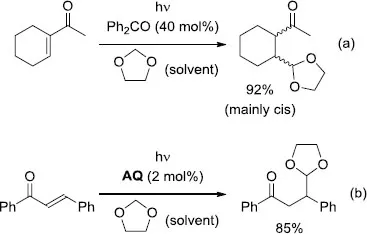
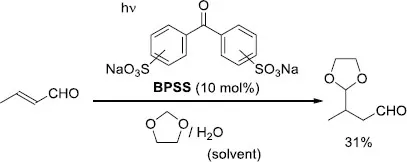
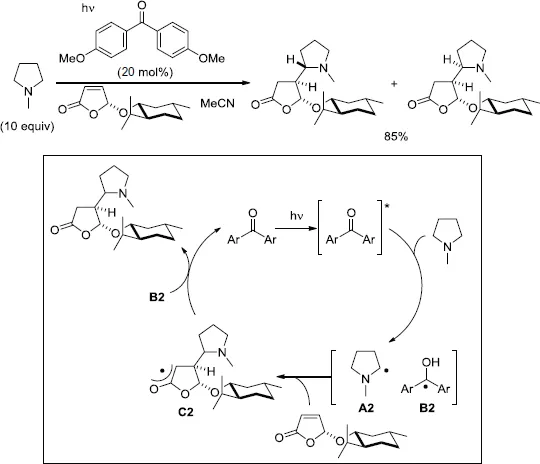
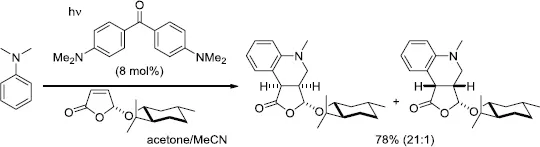
Table of contents
- Cover Page
- Title
- Copyright Page
- Preface
- About the Editors
- About the Contributors
- Contents
- Chapter 1 Ketones and Aldehydes
- Chapter 2 Quinones
- Chapter 3 Aromatics and Cyanoaromatics
- Chapter 4 Sulfur Heterocycles
- Chapter 5 Oxygen Heterocycles: Pyrylium Salts
- Chapter 6 Oxygen Heterocycles: Eosin Derivatives
- Chapter 7 Oxygen Heterocycles: Fluorescein, Rhodamines, Rose Bengal
- Chapter 8 Nitrogen Heterocycles: Porphyrins
- Chapter 9 Nitrogen Heterocycles: Acridinium Salts
- Chapter 10 Other Nitrogen Heterocycles: Carbazoles, Imides and PDI, mpg-C3N4, Tetrazines, Riboflavin, and BODIPY
- Chapter 11 Photoorganocatalysts for Polymerization
- Chapter 12 Photoorganocatalysis in Flow
- Index